
-
 America's Cup switches to two-year cycle
America's Cup switches to two-year cycle
-
Jesus could start for Arsenal in League Cup, says Arteta

-
 EU to probe Czech aid for two nuclear units
EU to probe Czech aid for two nuclear units
-
Strauss says sacking Stokes and McCullum will not solve England's Ashes woes

-
 Noel takes narrow lead after Alta Badia slalom first run
Noel takes narrow lead after Alta Badia slalom first run
-
Stocks diverge as rate hopes rise, AI fears ease

-
 Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
Man City players face Christmas weigh-in as Guardiola issues 'fatty' warning
-
German Christmas markets hit by flood of fake news

-
 Liverpool fear Isak has broken leg: reports
Liverpool fear Isak has broken leg: reports
-
West Indies captain says he 'let the team down' in New Zealand Tests

-
 Thailand says Cambodia agrees to border talks after ASEAN meet
Thailand says Cambodia agrees to border talks after ASEAN meet
-
Alleged Bondi shooters conducted 'tactical' training in countryside, Australian police say

-
 Swiss court to hear landmark climate case against cement giant
Swiss court to hear landmark climate case against cement giant
-
Knicks' Brunson scores 47, Bulls edge Hawks epic

-
 Global nuclear arms control under pressure in 2026
Global nuclear arms control under pressure in 2026
-
Asian markets rally with Wall St as rate hopes rise, AI fears ease

-
 Jailed Malaysian ex-PM Najib loses bid for house arrest
Jailed Malaysian ex-PM Najib loses bid for house arrest
-
Banned film exposes Hong Kong's censorship trend, director says

-
 Duffy, Patel force West Indies collapse as NZ close in on Test series win
Duffy, Patel force West Indies collapse as NZ close in on Test series win
-
Australian state pushes tough gun laws, 'terror symbols' ban after shooting

-
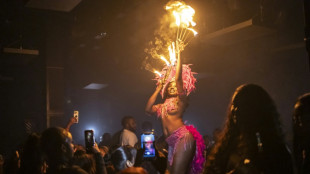 A night out on the town during Nigeria's 'Detty December'
A night out on the town during Nigeria's 'Detty December'
-
US in 'pursuit' of third oil tanker in Caribbean: official

-
 CO2 soon to be buried under North Sea oil platform
CO2 soon to be buried under North Sea oil platform
-
Steelers edge Lions as Bears, 49ers reach playoffs

-
 India's Bollywood counts costs as star fees squeeze profits
India's Bollywood counts costs as star fees squeeze profits
-
McCullum admits errors in Ashes preparations as England look to salvage pride

-
 Pets, pedis and peppermints: When the diva is a donkey
Pets, pedis and peppermints: When the diva is a donkey
-
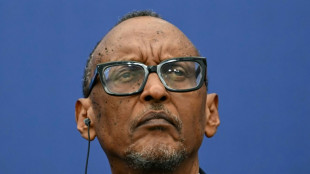
'A den of bandits': Rwanda closes thousands of evangelical churches
-
 Southeast Asia bloc meets to press Thailand, Cambodia on truce
Southeast Asia bloc meets to press Thailand, Cambodia on truce
-
As US battles China on AI, some companies choose Chinese

-
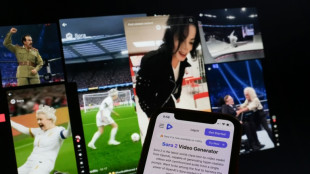 AI resurrections of dead celebrities amuse and rankle
AI resurrections of dead celebrities amuse and rankle
-
EonX Announces Update To Loan Facility

-
 Silver X Mining Announces Management Update
Silver X Mining Announces Management Update
-
Medicus Pharma Ltd. Announces Engagement With Reliant AI to Develop Artificial Intelligence (AI) Driven Clinical Data Analytics Platform

-
 Revolve Receives Generation Permit Approval for 130 MW El 24 Wind Project in Mexico
Revolve Receives Generation Permit Approval for 130 MW El 24 Wind Project in Mexico
-
NuRAN Restores Sites in Ghana and Resumes Network Deployment Activities in the Democratic Republic of the Congo

-
 1933 Industries Issues Final Reminder to 2024 Debenture Holders: December 22 Is the Deadline to Convert
1933 Industries Issues Final Reminder to 2024 Debenture Holders: December 22 Is the Deadline to Convert
-
Arrive AI to Attend CES 2026 to Engage Industry Leaders on the Future of Autonomous Delivery and AI-Driven Logistics

-
 Guanajuato Silver Receives TSXV Conditional Approval for Bolanitos Acquisition
Guanajuato Silver Receives TSXV Conditional Approval for Bolanitos Acquisition
-
Brenmiller Energy CEO Avi Brenmiller Issues Year-End Letter to Shareholders

-
 1933 Industries Achieves Positive Income in Q1 2026, Marking Third Consecutive Profitable Quarter
1933 Industries Achieves Positive Income in Q1 2026, Marking Third Consecutive Profitable Quarter
-
New Joint Initiative to Tackle Core Identity Challenge For The Global Precious Metals Industry

-
 Best Western Plus Lamplighter Inn & Conference Centre recognized with 2026 Consumer Choice Award for Hotel in London
Best Western Plus Lamplighter Inn & Conference Centre recognized with 2026 Consumer Choice Award for Hotel in London
-
CoTec Investment MagIron Acquires Reynolds Pellet Plant and Launches United States Based DR Grade Pellet and Merchant Pig Iron Strategy

-
 Saat & Saat Acquires Turkish Apparel Leader Aydinli Group, Expanding U.S. Polo Assn. Markets Across Turkey, the Middle East, Eastern Europe, and North Africa
Saat & Saat Acquires Turkish Apparel Leader Aydinli Group, Expanding U.S. Polo Assn. Markets Across Turkey, the Middle East, Eastern Europe, and North Africa
-
EON Resources Inc. Reports Management and Directors Buy an Additional 282,000 Shares of EON Class A Common Stock for a Total of 1,561,000 Shares Bought in 2025 and a Total Ownership of Over 5 million Shares

-
 Heirs Energies Agrees $750m Afreximbank Financing to Drive Long-Term Growth
Heirs Energies Agrees $750m Afreximbank Financing to Drive Long-Term Growth
-
Black Book Poll: "Governed AI" Emerges as the Deciding Factor in 2026 NHS Procurement

-
 Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares
Hemogenyx Pharmaceuticals PLC Announces Update on Admission of Shares
-
Pantheon Resources PLC Announces Shareholder Letter and Corporate Update on Dubhe-1


BioNxt Fast-Tracks U.S. Patent for MS Drug and Secures Broad Platform IP Covering Autoimmune Neurology Pipeline
VANCOUVER, BC / ACCESS Newswire / August 20, 2025 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTC PINK:BNXTF)(FSE:BXT), a bioscience company specializing in innovative drug delivery technologies, is pleased to announce two key milestones in the expansion and protection of its proprietary drug delivery platform targeting neurological autoimmune disorders. The Company has formally initiated the prioritized examination process for its U.S. patent application for BNT23001, a novel sublingual thin-film cladribine formulation for multiple sclerosis (MS), under the United States Patent and Trademark Office's (USPTO) Track One Program. Simultaneously, BioNxt has received acceptance of core claims for a broad international patent designed as a platform-level umbrella protecting the Company's sublingual thin-film delivery technology across a wide range of diseases, drug compounds, and applications.
Fast-Tracked U.S. Patent Filing via Track One to Accelerate Clinical Development Milestones
BioNxt is pursuing the USPTO's Track One Prioritized Examination Program to accelerate patent review for BNT23001. Once accepted, this program enables final patent disposition within 12 months, with most applicants receiving a first Office Action in approximately two months and a final decision in under seven months. For BioNxt, this fast-track status represents a strategic opportunity to secure near-term U.S. patent protection and strengthen its IP position ahead of pivotal bioequivalence studies and commercial partnering discussions.
BNT23001 is an orally dissolvable thin-film formulation of cladribine, a well-established immunomodulatory compound. Delivered sublingually, the formulation is designed for improved bioavailability, faster onset of action, and enhanced patient compliance, particularly in populations affected by dysphagia or seeking non-invasive alternatives to traditional tablets or injections.
A Strategic Path Toward Superbioavailability and Rapid Approval
BioNxt is advancing BNT23001 for relapsing-remitting MS (RRMS) and primary progressive MS (PPMS), as well as generalized myasthenia gravis (GMG). Merck KGaA is currently conducting a Phase 3 clinical trial to evaluate cladribine capsules in GMG (ClinicalTrials.gov: NCT06463587), further validating the therapeutic relevance of the compound beyond MS. This clinical activity by Merck reinforces the therapeutic relevance of cladribine in GMG and underscores the opportunity for BioNxt to differentiate and potentially partner or collaborate with its own next-generation, patient-centric delivery format.
BioNxt's objective is to confirm that its sublingual thin film offers superbioavailability in comparison to Merck's MAVENCLAD® tablets, which generated more than US$1 billion in sales in 2023. If superior bioavailability is demonstrated relative to MAVENCLAD®, BioNxt would qualify for an accelerated regulatory path under Article 10a of the European hybrid application framework, requiring only comparative PK data in healthy volunteers. A preclinical pharmacokinetic (PK) study in pigs, an established translational model for oral and sublingual absorption, will be initiated in the near-term to provide critical bioavailability data to support regulatory positioning and formulation optimization.
BioNxt's Broad Patent: A Platform-Level Umbrella for Autoimmune Neurology
In parallel to its U.S. patent fast-tracking, BioNxt has achieved a major intellectual property milestone with the acceptance of core claims in its broad international umbrella patent protecting its thin-film delivery platform. This foundational patent is not limited to BNT23001, but instead provides platform-level protection for a wide range of drug compounds, therapeutic indications, administration methods, and manufacturing processes.
The umbrella patent provides long-term exclusivity, extending into the 2040s, not only for cladribine, but for a broad class of drug compounds delivered via BioNxt's proprietary sublingual thin-film technology. This includes protection for composition, manufacturing, administration methods, and use across multiple autoimmune and neuroinflammatory indications.
Key disease targets include relapsing-remitting and primary progressive multiple sclerosis, generalized myasthenia gravis, neuromyelitis optica spectrum disorders (NMOSD), autoimmune encephalitis, central nervous system (CNS) vasculitis, optic neuritis, Hashimoto's encephalopathy, cerebral lupus, and Sjögren's syndrome. While Sjögren's is primarily known for its effects on exocrine glands, it is also associated with neurological complications in some patients.
The patent has received core claim acceptance from both the European Patent Office (EPO) and the Eurasian Patent Organization (EAPO), and BioNxt has initiated national-phase filings in the United States, Canada, Japan, Australia, and New Zealand. This patent framework enables BioNxt to scale its platform across additional indications using the same delivery modality, reducing development timelines and leveraging shared regulatory infrastructure.
Targeting Global Markets and Significant Unmet Need
Autoimmune neurodegenerative diseases represent a significant global burden with limited therapeutic options and poor adherence to existing therapies. BioNxt's thin-film delivery technology is designed to improve tolerability and usability across these patient populations. Unlike conventional tablets or injectable formulations, BioNxt's thin-film platform bypasses the gastrointestinal tract, supports rapid absorption, and improves dosing convenience, critical in chronic autoimmune disorders where long-term adherence is essential.
More than 2.8 million people are living with multiple sclerosis (MS) globally, including nearly 1 million in the United States alone, according to the National Multiple Sclerosis Society and a 2019 study published in Neurology (Wallin et al., 2019; National MS Society). The U.S. MS drug market was valued at USD 7.81 billion in 2024 and is forecast to grow to over USD 17.15 billion by 2034, reflecting a CAGR of 8.18% (Precedence Research, 2024).
Generalized myasthenia gravis affects an estimated 700,000 people globally and remains an area of unmet therapeutic need (ClinicalTrialsArena, 2023). BioNxt estimates that its initial target indications, MS and GMG, represent a combined total addressable market of USD 42-46 billion by 2032, based on internal analysis and third-party forecasting. The central nervous system therapeutics market is projected to exceed USD 239 billion by 2032, highlighting the broader opportunity for innovation in neuroimmunology (GMI Insights, 2024).
The Company expects to report results from its preclinical PK study later this year, supporting its regulatory strategy and partnership discussions.
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next-generation drug delivery platforms, diagnostic screening systems, and active pharmaceutical ingredient development. Its proprietary platforms include sublingual thin films, transdermal patches, oral tablets, and a new targeted chemotherapy platform designed to deliver cancer drugs directly to tumors while reducing side effects.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient-centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co-Founder, CEO and Director
Email: [email protected]
Phone: +1 604.250.6162
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt-solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward-Looking" Information
This press release contains "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian securities laws (collectively, "forward-looking information"). Forward-looking information includes, but is not limited to, statements regarding the anticipated grant and scope of European and Eurasian patent rights; the Company's plans for additional international patent filings; the development, clinical evaluation, and commercialization of the Company's Cladribine thin-film (BNT23001) for multiple sclerosis; the strategic importance of intellectual property; the timing and outcome of regulatory processes; and the potential expansion of BioNxt's sublingual drug delivery platform to other therapeutic areas.
Forward-looking information is based on management's reasonable assumptions, expectations, estimates, and projections as of the date of this press release. These statements are subject to known and unknown risks, uncertainties, and other factors-many of which are beyond the Company's control-that may cause actual results, performance, or achievements to differ materially from those expressed or implied by such forward-looking information. These risks and uncertainties include, but are not limited to: patent examination and prosecution outcomes; changes in regulatory or legal frameworks; clinical trial results; scalability of manufacturing processes; strategic partnership risks; and broader economic, financial, or geopolitical conditions.
Readers are cautioned not to place undue reliance on forward-looking information. Although the Company believes that the expectations and assumptions reflected in such statements are reasonable, there can be no assurance that they will prove to be correct. Except as required by applicable securities laws, BioNxt undertakes no obligation to update or revise any forward-looking information, whether as a result of new information, future events, or otherwise.
Mavenclad® is a registered trademark of Merck KGaA, Darmstadt, Germany. It is not affiliated with or endorsed by BioNxt Solutions Inc.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
C.Garcia--AMWN
