
-
 Moldova's pro-EU party hails poll win despite 'dirty' Russian tactics
Moldova's pro-EU party hails poll win despite 'dirty' Russian tactics
-
Typhoon Bualoi kills dozens in Vietnam and Philippines

-
 Wallabies' big-man Skelton ready to impose himself against All Blacks
Wallabies' big-man Skelton ready to impose himself against All Blacks
-
Robertson wants All Blacks to 'pressure' Wallabies in rematch

-
 Sinner cruises into China Open semi-finals as Swiatek moves on
Sinner cruises into China Open semi-finals as Swiatek moves on
-
GSK switches CEO as Trump tariffs test pharma

-
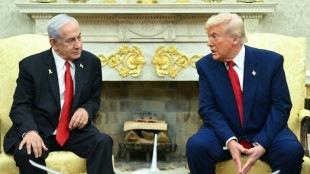 Trump to push Netanyahu on Gaza peace plan at White House
Trump to push Netanyahu on Gaza peace plan at White House
-
Most markets track Wall St gains after US inflation data

-
 Typhoon Bualoi batters Vietnam coast, killing 11
Typhoon Bualoi batters Vietnam coast, killing 11
-
Germany's Lufthansa to slash 4,000 jobs by 2030

-
 Gunman kills four in attack on northern US Mormon church
Gunman kills four in attack on northern US Mormon church
-
Moldova's pro-EU party wins key polls after Russian meddling claims

-
 Mourinho Chelsea return prompts old memories, mixed feelings
Mourinho Chelsea return prompts old memories, mixed feelings
-
'Predators': how reality TV explains Epstein obsession

-
 Most Asian markets track Wall St higher after US inflation data
Most Asian markets track Wall St higher after US inflation data
-
India, Pakistan trade accusations after Asia Cup trophy debacle

-
 Power-packed Australia favourites to rewrite World Cup history
Power-packed Australia favourites to rewrite World Cup history
-
Latin artist Bad Bunny to headline Super Bowl half-time show

-
 Air France, Airbus back on trial over doomed 2009 Rio flight
Air France, Airbus back on trial over doomed 2009 Rio flight
-
India's divine designs meld with AI at Durga Puja festival

-
 Donald won't rule out Ryder Cup captain return after Europe win
Donald won't rule out Ryder Cup captain return after Europe win
-
Who is Matthieu Blazy, the new man at Chanel?

-
 'New chapter': Paris Fashion Week to showcase industry makeover
'New chapter': Paris Fashion Week to showcase industry makeover
-
Bradley on US Ryder Cup loss: 'This is no one's fault but mine'

-
 Four killed in attack on northern US Mormon church
Four killed in attack on northern US Mormon church
-
Bradley calls for Ryder Cup rule change for injuries

-
 McIlroy slams 'unacceptable' Ryder Cup heckling
McIlroy slams 'unacceptable' Ryder Cup heckling
-
Embattled Australia telco giant hit by another major outage

-
 American Critical Minerals Confirms Annual Renewal of all Potash Licenses and Lithium Claims across the Green River Project
American Critical Minerals Confirms Annual Renewal of all Potash Licenses and Lithium Claims across the Green River Project
-
BioNxt Reports Sublingual Cladribine Multiple Sclerosis Drug Milestones Including Large-Animal Study for Validation and Dosing Optimization

-
 Angle PLC Announces Presentation of Data on Glioblastoma
Angle PLC Announces Presentation of Data on Glioblastoma
-
Guardian Metal Resources PLC Announces Pilot North: Very-High Grade Assay Results

-
 31 Concept Accelerates Next-Gen DPI Leadership With Strategic Acquisition of Xynthor AI
31 Concept Accelerates Next-Gen DPI Leadership With Strategic Acquisition of Xynthor AI
-
Onco-Innovations Advances Preclinical Program as Nucro-Technics Begins Analytical Development for PNKP Technology

-
 Mahomes leads resurgent Chiefs in Ravens rout, Eagles stay unbeaten
Mahomes leads resurgent Chiefs in Ravens rout, Eagles stay unbeaten
-
Moldova's pro-EU party tops polls hit by Russian meddling claims

-
 Europe win emotional Ryder Cup triumph after US fightback
Europe win emotional Ryder Cup triumph after US fightback
-
Two dead after shooting, fire at US Mormon church

-
 Europe must step up efforts to protect environment: report
Europe must step up efforts to protect environment: report
-
Eagles down Bucs to stay unbeaten, Bills march on

-
 Incumbent absent as Cameroon presidential campaigning picks up
Incumbent absent as Cameroon presidential campaigning picks up
-
AC Milan beat champions Napoli to make Serie A title statement

-
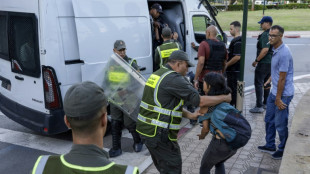 Scores arrested on second day of Morocco protests: NGO
Scores arrested on second day of Morocco protests: NGO
-
India beat Pakistan for Asia Cup title but skip trophy presentation

-
 'One Battle After Another' debuts top of N. America box office
'One Battle After Another' debuts top of N. America box office
-
Two dead after US shooting, fire at Mormon church

-
 Mitchell open to coaching first Women's Lions in 2027
Mitchell open to coaching first Women's Lions in 2027
-
Vagnoman sends Stuttgart past Cologne in Bundesliga

-
 Stars turn out for Armani's final collection in Milan
Stars turn out for Armani's final collection in Milan
-
Massive Russian drone and missile attack kills four in Kyiv


BioNxt Reports Sublingual Cladribine Multiple Sclerosis Drug Milestones Including Large-Animal Study for Validation and Dosing Optimization
VANCOUVER,BC / ACCESS Newswire / September 29, 2025 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTCQB:BNXTF)(FSE:BXT), a bioscience company specializing in innovative drug delivery technologies, is pleased to provide an update on its proprietary Cladribine sublingual thin-film drug reformulation program (BNT23001), a next-generation delivery platform designed to improve bioavailability, patient adherence, and therapeutic outcomes in the treatment of neurological disorders, primarily multiple sclerosis (MS).
Cladribine Sublingual Thin-Film Program: Manufacturing and Development Milestones
BioNxt has successfully completed key technology transfers to its European contract development and manufacturing organization ("CDMO"), including both the process transfer and the analytical method transfer. These achievements represent critical steps toward ensuring reproducibility, scalability, and regulatory compliance in the production of the Company's pharmaceutical-grade sublingual thin-film dosage forms.
In parallel, BioNxt confirms that the active pharmaceutical ingredient (API) for Cladribine has been ordered and is currently in transit to the CDMO for use in the manufacturing of a pilot batch. This batch will be used to produce clinical-grade thin film products, advancing the program toward its next preclinical and clinical phases.
The Company is now preparing for the optimization and validation of analytical methods, together with the final refinement of product and process parameters at its CDMO partner. These activities will enable the production of sublingual thin-film samples for a large-animal (pig) bioavailability study, scheduled to begin in October 2025. Pigs are widely recognized as a translationally relevant model for human gastrointestinal absorption, and this study is specifically designed to confirm and strengthen the highly promising bioavailability results demonstrated in earlier animal studies. A successful outcome will provide robust, clinically translatable evidence of the formulation's performance, establishing the final preclinical validation step and dosage optimization prior to human evaluation.
Building on this foundation, BioNxt plans to manufacture a pilot batch for a first-in-human clinical pilot study, targeted for Q1 2026. This milestone trial would represent a major inflection point in the clinical development of BioNxt's Cladribine sublingual reformulation.
Clinical and Market Impact
BioNxt's Cladribine sublingual thin-film program, BNT23001, represents a novel approach to oral drug delivery in neurological disease, with the potential to improve patient outcomes through enhanced pharmacokinetics and ease of administration. The program also complements BioNxt's growing portfolio of proprietary sublingual thin-film technologies targeting high-value pharmaceutical markets.
"With both our development program and global patent strategy advancing on schedule, BioNxt is steadily building the foundation for clinical translation and commercial success," said Hugh Rogers, CEO of BioNxt. "Cladribine represents a powerful therapeutic option with untapped potential, and our sublingual thin-film reformulation may unlock significant advantages in terms of bioavailability, patient adherence, and market accessibility. The progress we are reporting today reinforces our conviction in the program's importance as a core pillar of BioNxt's commercialization pipeline."
Next Steps
Completion of analytical optimization and validation at the European CDMO;
Final process and product optimization, followed by clinical sample production;
Initiation of a large-animal bioavailability study in October 2025, expected to be the final preclinical study before human trials;
Preparation for the manufacture of clinical samples for the first-in-human clinical study in Q1 2026; and
Ongoing patent nationalizations and accelerated US patent review under Track One.
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next-generation drug delivery platforms, diagnostic screening systems, and active pharmaceutical ingredient development. Its proprietary platforms include sublingual thin films, transdermal patches, oral tablets, and a new targeted chemotherapy platform designed to deliver cancer drugs directly to tumors while reducing side effects.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient-centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co-Founder, CEO and Director
Email: [email protected]
Phone: +1 604.250.6162
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt-solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward-Looking" Information
This press release contains "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian securities laws (collectively, "forward-looking information"). Such information may include, but is not limited to, statements regarding: the anticipated grant, scope, and timing of European, Eurasian, and other international patent rights; the Company's plans for additional national filings; the development, clinical evaluation, regulatory approval, and commercialization of the Company's Cladribine sublingual thin-film (BNT23001) for multiple sclerosis; the strategic importance of intellectual property protection; the timing, cost, and outcome of preclinical and clinical studies; and the potential application of BioNxt's sublingual thin-film drug delivery platform across additional therapeutic areas.
Forward-looking information is based on management's current expectations, assumptions, estimates, and projections as of the date of this press release. Such statements are subject to inherent risks and uncertainties, many of which are beyond the Company's control, that could cause actual results, performance, or achievements to differ materially from those expressed or implied. These risks and uncertainties include, but are not limited to: outcomes of patent examination and prosecution processes; changes in regulatory requirements or legal frameworks; the results, timing, and costs of preclinical and clinical studies; scalability and reproducibility of manufacturing processes; the availability of strategic partnerships and funding; and broader economic, financial, or geopolitical factors.
Readers are cautioned not to place undue reliance on forward-looking information. Although the Company believes the expectations and assumptions underlying such information are reasonable, there can be no assurance that they will prove to be correct. Except as required under applicable securities laws, BioNxt undertakes no obligation to update or revise any forward-looking information, whether as a result of new information, future events, or otherwise.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
O.Karlsson--AMWN