
-
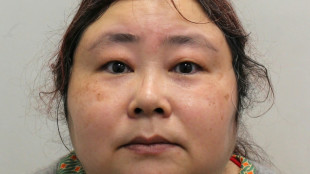 UK court jails Chinese bitcoin fraudster for over 11 years
UK court jails Chinese bitcoin fraudster for over 11 years
-
Fanfare as Guinea launches enormous Simandou iron ore mine

-
 Iraqis vote in general election at crucial regional moment
Iraqis vote in general election at crucial regional moment
-
Shock follows carnage after suicide bombing in Islamabad

-
 Ford returns to pull England strings against All Blacks
Ford returns to pull England strings against All Blacks
-
Stocks mixed as end to US shutdown appears closer

-
 BBC must 'fight' for its journalism, outgoing chief says amid Trump lawsuit threat
BBC must 'fight' for its journalism, outgoing chief says amid Trump lawsuit threat
-
Atalanta turn to Palladino after Juric sacking

-
 'Sayyid says': Influential Shiite cleric's supporters boycott Iraq vote
'Sayyid says': Influential Shiite cleric's supporters boycott Iraq vote
-
'It's un-British': lawmakers raise concerns about aquarium penguins

-
 Prosecutor files 142 charges against Istanbul mayor, a top Erdogan critic
Prosecutor files 142 charges against Istanbul mayor, a top Erdogan critic
-
Agha hundred lifts Pakistan to 299-5 in 1st Sri Lanka ODI

-
 German court rules against OpenAI in copyright case
German court rules against OpenAI in copyright case
-
Calls for 'mano dura' as crime-rattled Chile votes for president

-
 Pakistani Taliban claim deadly suicide attack in Islamabad
Pakistani Taliban claim deadly suicide attack in Islamabad
-
BBC grapples with response to Trump legal threat

-
 Cristiano Ronaldo says 2026 World Cup 'definitely' his last
Cristiano Ronaldo says 2026 World Cup 'definitely' his last
-
Trump says 'we've had a lot of problems' with France

-
 Stocks mostly rise as end to US shutdown appears closer
Stocks mostly rise as end to US shutdown appears closer
-
'Splinternets' threat to be avoided, says web address controller

-
 Yamal released from World Cup qualifiers by 'upset' Spanish federation
Yamal released from World Cup qualifiers by 'upset' Spanish federation
-
China's 'Singles Day' shopping fest loses its shine for weary consumers

-
 Suicide bombing in Islamabad kills 12, wounds 27
Suicide bombing in Islamabad kills 12, wounds 27
-
Philippines digs out from Typhoon Fung-wong as death toll climbs

-
 Iraqis vote in general election at a crucial regional moment
Iraqis vote in general election at a crucial regional moment
-
Asian stocks wobble as US shutdown rally loses steam

-
 UK unemployment jumps to 5% before key govt budget
UK unemployment jumps to 5% before key govt budget
-
Japanese 'Ran' actor Tatsuya Nakadai dies at 92

-
 AI stock boom delivers bumper quarter for Japan's SoftBank
AI stock boom delivers bumper quarter for Japan's SoftBank
-
Asian stocks struggle as US shutdown rally loses steam

-
 India probes deadly Delhi blast, vows those responsible will face justice
India probes deadly Delhi blast, vows those responsible will face justice
-
Pistons win streak hits seven on night of NBA thrillers

-
 US state leaders take stage at UN climate summit -- without Trump
US state leaders take stage at UN climate summit -- without Trump
-
Burger King to enter China joint venture, plans to double stores

-
 Iraqis vote in general election in rare moment of calm
Iraqis vote in general election in rare moment of calm
-
Philippines digs out from Typhoon Fung-wong as death toll climbs to 18

-
 'Demon Slayer' helps Sony hike profit forecasts
'Demon Slayer' helps Sony hike profit forecasts
-
Who can qualify for 2026 World Cup in next round of European qualifiers

-
 Ireland's climate battle is being fought in its fields
Ireland's climate battle is being fought in its fields
-
Sony hikes profit forecasts on strong gaming, anime sales

-
 End to US government shutdown in sight as stopgap bill advances to House
End to US government shutdown in sight as stopgap bill advances to House
-
'Western tech dominance fading' at Lisbon's Web Summit

-
 Asian stocks rise as record US shutdown nears end
Asian stocks rise as record US shutdown nears end
-
'Joy to beloved motherland': N.Korea football glory fuels propaganda

-
 Taiwan coastguard faces China's might near frontline islands
Taiwan coastguard faces China's might near frontline islands
-
Concentration of corporate power a 'huge' concern: UN rights chief

-
 Indian forensic teams scour deadly Delhi car explosion
Indian forensic teams scour deadly Delhi car explosion
-
Trump says firebrand ally Greene has 'lost her way' after criticism

-
 Show shines light on Mormons' unique place in US culture
Show shines light on Mormons' unique place in US culture
-
Ukraine, China's critical mineral dominance, on agenda as G7 meets


Dogwood Announces Enrollment of First 100 Patients in Ongoing Halneuron(R) Phase 2b Trial, Interim Sample Size Analysis on Track for December 2025
- Continued low early termination rate among the first 80 study completers suggests Halneuron® and placebotreatmenthave been well tolerated
ATLANTA, GEORGIA / ACCESS Newswire / November 11, 2025 / Dogwood Therapeutics, Inc. (Nasdaq:DWTX) (the "Company"), a development-stage biotechnology company developing new medicines to treat pain and neuropathy, today announced it has successfully enrolled the first 100 patients in its ongoing HALT-CINP Phase 2b CINP trial. HALT-CINP remains on track to conduct a prespecified interim analysis during the fourth quarter of 2025 on patients who have completed or been terminated from the four-week study.
"High interest among cancer patients, significant unmet medical need and positive word of mouth among patients who have participated in the study have contributed to the brisk pace of recruitment in this landmark chemotherapy-induced neuropathic pain study," said R. Michael Gendreau, M.D., Ph.D., Dogwood Therapeutics Chief Medical Officer. "The primary goal of our fourth quarter interim analysis is to evaluate and adjust, if needed, the Phase 2b study sample size required to demonstrate statistically significant results. We are currently continuing with the original plan for a 200-patient sample size for this study, with final data available by the middle of 2026."
Halneuron® CINP Phase 2b Trial ("HALT-CINP") Overview (NCT06848348)
HALT-CINPis a randomized, phase 2b clinical trial evaluating the safety and effectiveness of Halneuron® versus placebo in cancer patients with established neuropathy due to a previous platinum or taxane based chemotherapy regimen. Participants receive 8 sub-cutaneous doses of Halneuron® or placebo over a 14-day period and will be followed for a total of 28 days for safety and effectiveness. The primary endpoint for this study is the change from baseline to week four in the weekly average of daily 24-hour recall pain intensity scores. The study is being conducted at approximately 25 sites in the US. Secondary measures will assess Halneuron's® treatment effects on sleep, fatigue, neuropathy symptoms and overall patient health.
About Dogwood Therapeutics
Dogwood Therapeutics (Nasdaq: DWTX) is a development-stage biopharmaceutical company focused on developing new medicines to treat pain and neuropathic disorders. The Dogwood research pipeline includes two first-in-class development candidates, Halneuron® and SP16 IV.
Our lead product candidate, Halneuron®, is in Phase 2b development to treat pain conditions including the neuropathic pain associated with chemotherapy treatment. Halneuron® has been granted fast track designation from the Food and Drug Administration ("FDA") for the treatment of CINP. Halneuron® is a non-opioid, NaV 1.7 analgesic which is a highly specific voltage-gated sodium channel modulator, a mechanism known to be effective for reducing pain transmission. In clinical studies, Halneuron® treatment has demonstrated pain reduction in pain related to general cancer and in pain related to chronic chemotherapy-induced neuropathic pain ("CINP").
SP16 IV is a low-density lipoprotein receptor related protein-1 (LRP1) agonist with potential to treat neuropathy and prevent or repair nerve damage following chemotherapy. SP16 acts as an LRP1 agonist that in turn provides alpha-1-antitrypsin-like activity. Consistent with alpha-1-antitrypsin anti-inflammatory and immunomodulatory actions, SP16 preclinically demonstrated anti-inflammatory (analgesic) action via potential reductions in IL-6, IL-8, IL1B and TNF-alpha levels, as well as potential to repair damaged tissue via increases in pAKT and pERK that regulate fundamental processes like growth, proliferation and survival. The forthcoming SP16 IV Phase 1b CINP trial is fully funded by the National Cancer Institute.
For more information, please visit www.dwtx.com.
Forward-Looking Statements:
Statements in this press release contain "forward-looking statements," within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "suggest," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on Dogwood's current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including risks related to the completion, timing and results of current and future clinical studies relating to Dogwood's product candidates. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled "Risk Factors" in the Annual Report on Form 10-K for the year ended December 31, 2024, which has been filed with the Securities and Exchange Commission. Forward-looking statements contained in this announcement are made as of this date, and Dogwood undertakes no duty to update such information except as required under applicable law.
Investor Relations:
CORE IR
(516) 222-2560
[email protected]
SOURCE: Dogwood Therapeutics, Inc.
View the original press release on ACCESS Newswire
A.Jones--AMWN
