
-
 Pogacar plays down yellow jersey after Evenepoel wins Tour time trial
Pogacar plays down yellow jersey after Evenepoel wins Tour time trial
-
Macron, Starmer talk Channel migration as UK visit gets political

-
 Sinner powers into Wimbledon semi-finals to ease injury fears
Sinner powers into Wimbledon semi-finals to ease injury fears
-
Angel Correa leaves Atletico for Mexican club Tigres

-
 Thunder's Holmgren agrees to contract extension worth up to $250 mn: reports
Thunder's Holmgren agrees to contract extension worth up to $250 mn: reports
-
Musk's AI chatbot under fire for posts praising Hitler

-
 Evenepoel triumphs in Tour de France time trial as Pogacar slips into yellow
Evenepoel triumphs in Tour de France time trial as Pogacar slips into yellow
-
Trump issues more letters to countries in push for tariff deals

-
 Fears grow that Texas floods death toll could surge
Fears grow that Texas floods death toll could surge
-
Yemen's Huthis claim deadly Red Sea attack on merchant ship

-
 Putellas going with flow in dominant Spain's Euro 2025 charge
Putellas going with flow in dominant Spain's Euro 2025 charge
-
Copper giant Chile awaits 'official' news on US tariff raise

-
 Pant says keeping to Bumrah even tougher than facing the India star
Pant says keeping to Bumrah even tougher than facing the India star
-
X chief Yaccarino steps down after two years

-
 Trump hosts African leaders in landmark trade-focused summit
Trump hosts African leaders in landmark trade-focused summit
-
Greece to halt asylum hearings for migrants on boats from Africa

-
 Ex-Real Madrid coach Ancelotti gets year's jail for tax fraud
Ex-Real Madrid coach Ancelotti gets year's jail for tax fraud
-
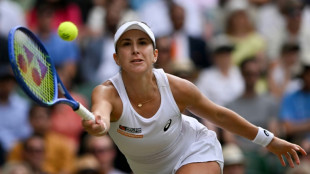
Bencic beats Andreeva to reach first Wimbledon semi-final
-
 Fears grow that Texas floods death toll could still surge
Fears grow that Texas floods death toll could still surge
-
Six rescued from cargo ship attacked in Red Sea: EU naval force

-
 Searching for Grandma Alicia after Texas floods
Searching for Grandma Alicia after Texas floods
-
Lyon stave off relegation after successful appeal

-
 Israel FM says Hamas truce deal 'achievable' despite hurdles in talks
Israel FM says Hamas truce deal 'achievable' despite hurdles in talks
-
Christian Horner - a brutal end to a rollercoaster reign at Red Bull

-
 Swiatek gets 'goosebumps' after reaching first Wimbledon semi-final
Swiatek gets 'goosebumps' after reaching first Wimbledon semi-final
-
Zelensky talks peace with pope ahead of Ukraine conference

-
 Christian Horner - a brutal end to a spicy reign at Red Bull
Christian Horner - a brutal end to a spicy reign at Red Bull
-
Dozens of sites vie for UNESCO world heritage list spot

-
 Swiatek into first Wimbledon semi-final
Swiatek into first Wimbledon semi-final
-
Syrian designer Rami Al Ali to make history at Paris Couture Week

-
 'Hothead' Fognini announces retirement from tennis
'Hothead' Fognini announces retirement from tennis
-
Werner unveiled as first new Leipzig coach in Klopp era

-
 Zelensky talks peace with pope ahead of Ukraine recovery conference
Zelensky talks peace with pope ahead of Ukraine recovery conference
-
Musk's chatbot Grok slammed for praising Hitler, dishing insults

-
 Another Lions injury worry after fullback Kinghorn limps off
Another Lions injury worry after fullback Kinghorn limps off
-
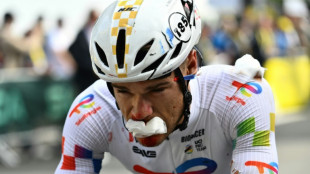
Rider quits Tour de France after cycling 174km with fractured shoulder
-
 Top European rights court finds Russia committed abuses in Ukraine
Top European rights court finds Russia committed abuses in Ukraine
-
Inspired Queensland upset NSW to snatch State of Origin crown

-
 Lions tame gutsy Brumbies for fourth straight win on Australia tour
Lions tame gutsy Brumbies for fourth straight win on Australia tour
-
Red Bull sack F1 team chief Horner

-
 Demna bows out at Balenciaga with star-studded Paris catwalk show
Demna bows out at Balenciaga with star-studded Paris catwalk show
-
Lions tame gutsy Brumbies to make it four straight wins

-
 Djokovic eyes Wimbledon history, wounded Sinner in spotlight
Djokovic eyes Wimbledon history, wounded Sinner in spotlight
-
European stocks brush off Trump's copper, pharma tariff threats

-
 France police raid far-right party offices over campaign financing
France police raid far-right party offices over campaign financing
-
Commerzbank commits to strategy as UniCredit ups direct stake

-
 Deadly temperatures blasted western Europe in record hot June
Deadly temperatures blasted western Europe in record hot June
-
Volkswagen US deliveries fall as Trump tariffs bite

-
 England recall Archer after injury exile for third Test against India
England recall Archer after injury exile for third Test against India
-
Red Bull sack team chief Horner after two decades in charge


ZEO ScientifiX Positions for National Growth as Stem Cell Market Opens in Florida
ZEO Launches New Website and Agency Partnerships to Address Expected Biologics Market Demand Following Florida's New Stem Cell Law
FORT LAUDERDALE, FL / ACCESS Newswire / July 9, 2025 / ZEO ScientifiX, Inc. ("ZEO" or the "Company") (OTCQB:ZEOX), a clinical-stage biopharmaceutical company pioneering the development of innovative biological therapeutics, is spearheading an expansion initiative aligned with the implementation of Florida's new SB-1768 stem cell law. With Florida now authorizing the use of non-FDA-approved stem cell therapies that are compliant with the new regulations for the treatment of certain defined conditions, ZEO is seeking to take advantage of the opportunity to set what it believes will be the industry standard through its product platform, new agency partnerships and educational initiatives.
The Future of Regenerative Medicine is Now
ZEO's turnkey product platform consists of a proprietary suite of ethically sourced biologics, developed and processed in a state-of-the-art FDA-registered, cGMP-compliant facility and physician education initiatives to assist Florida physicians in delivering regenerative care safely and compliantly under the new Florida guidelines. With an inventory of safe, regulatory-compliant biologics, a history of first-class research and competitive pricing, ZEO believes it is uniquely positioned to lead Florida's new era of accessibility and innovation in the biologics market.
"We believe that ZEO is entering into a new chapter of growth and expect additional states to join Florida in recognizing the therapeutic potential of regenerative biologics," said Ian Bothwell, interim chief executive officer of ZEO ScientifiX. "We're not just ready for this shift, we've built the product platform, partnerships and scientific roadmap to lead it."
With an expanding product pipeline, physician education initiatives and a strong compliance focus, ZEO is positioning itself as the go-to source for safe, effective and ethically developed regenerative therapies that have been legally approved.
ZEO Launches New Website to Reflect Market Evolution
In conjunction with the July 1, 2025, effective date of the new Florida legislation, ZEO has unveiled a newly redesigned website at www.zeoscientifix.com. The new platform highlights the Company's commitment to education, compliance and long-term therapeutic innovation - offering streamlined access to clinical data, physician resources and product information.
Strategic Agency Partnerships Support National Brand Presence
To further support this next phase of growth, ZEO ScientifiX has partnered with two key agencies:
Plus4 Public Relations, a national firm specializing in earned media, content development and strategic communications;
Small Circle, a full-service creative agency focused on brand identity, design and audience resonance.
ZEO believes that these partnerships will help them amplify their message and elevate their presence across emerging regenerative medicine markets in Florida and other states, when and where applicable.
About ZEO ScientifiX, Inc.
ZEO ScientifiX, Inc. (OTCQB:ZEOX) is not just committed to maintaining pace with regulatory change - our goal is to set the standard. As a clinical-stage biopharmaceutical company focused on the development of biological therapeutic platforms, we believe that when physicians are educated, patients are better served - and innovation thrives. We are driven by a commitment to advance the frontiers of regenerative medicine and biologic therapeutics, delivering meaningful solutions for patients and providers worldwide. Our proprietary products, including (a) Zofin™, which are derived from perinatal sources and manufactured to retain the naturally occurring extracellular vesicles, proteins and cell secreted nanoparticles, and (b) Patient Pure X™ ("PPX™"), an autologous biologic containing a nanoparticle fraction that is precipitated from a patient's own peripheral blood, are manufactured in an FDA-registered, cGMP-compliant laboratory. To learn more, please visit https://zeoscientifix.com.
Forward-Looking Statements
Certain statements contained in this press release should be considered forward-looking statements within the meaning of the Securities Act of 1933, as amended (the "Securities Act"), the Securities Exchange Act of 1934, as amended (the "Exchange Act"), and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are often identified by the use of forward-looking terminology such as "will," "believes," "expects," "potential," or similar expressions, involving known and unknown risks and uncertainties. Although the Company believes that the expectations reflected in these forward-looking statements are reasonable, they do involve assumptions, risks, and uncertainties, and these expectations may prove to be incorrect. No assurances can be given that the Florida Stem Cell Law will be beneficial to the Company or any initiatives related to this new law will increase our revenues and/or the price of our common stock to a level that is attractive to brokerage houses and institutional investors. We remind you that actual results could vary dramatically as a result of known and unknown risks and uncertainties, including but not limited to: potential issues related to our financial condition, competition, the ability to retain key personnel, product safety, efficacy and acceptance, the commercial success of any new products or technologies, success of clinical programs, ability to retain key customers, our inability to expand sales and distribution channels, legislation or regulations affecting our operations, including product pricing, reimbursement or access, the ability to protect our patents and other intellectual property both domestically and internationally, and other known and unknown risks and uncertainties, including the risk factors discussed in the Company's periodic reports that are filed with the SEC and available on the SEC's website (http://www.sec.gov). You are cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements attributable to the Company or persons acting on its behalf are expressly qualified in their entirety by these risk factors. Specific information included in this press release may change over time and may or may not be accurate after the date of the release. ZEO has no intention and specifically disclaims any duty to update the information in this press release.
Contact Information
Chloe Detrick
Plus4 Public Relations
[email protected]
SOURCE: ZEO ScientifiX, Inc.
View the original press release on ACCESS Newswire
F.Dubois--AMWN