
-
 El Salvador rights group says forced out by Bukele 'repression'
El Salvador rights group says forced out by Bukele 'repression'
-
US may revise hormone replacement therapy warnings

-
 US House passes landmark crypto measures in win for Trump
US House passes landmark crypto measures in win for Trump
-
Trump diagnosed with vein issue after leg swelling and hand bruising

-
 England reach Euro 2025 semis after shootout win over Sweden
England reach Euro 2025 semis after shootout win over Sweden
-
Netflix profits surge off ads, higher subscription prices

-
 US stocks end at fresh records as markets shrug off tariff worries
US stocks end at fresh records as markets shrug off tariff worries
-
British Open round 1: Who said what

-
 Former Springbok Ackermann succeeds White as Bulls coach
Former Springbok Ackermann succeeds White as Bulls coach
-
Milei steps up attacks on media as election nears

-
 Netflix profits surge 45% off higher subscription prices
Netflix profits surge 45% off higher subscription prices
-
McIlroy pushed to solid British Open start by home support

-
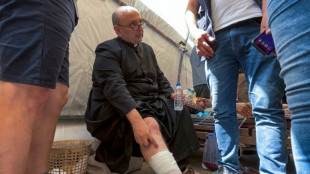 Israel PM voices regret after three killed at Catholic church in Gaza
Israel PM voices regret after three killed at Catholic church in Gaza
-
Scheffler makes bright British Open start, McIlroy three shots back

-
 Fraud probe opened into Mbappe payments to police officers
Fraud probe opened into Mbappe payments to police officers
-
Trump diagnosed with vein issue after leg swelling, hand bruising

-
 US authorizes Juul to market vaping products
US authorizes Juul to market vaping products
-
Pacquiao, 46, eyes comeback upset in Barrios showdown

-
 Austrian space diver Felix Baumgartner was 'born to fly'
Austrian space diver Felix Baumgartner was 'born to fly'
-
Slashed US aid showing impact, as Congress codifies cuts

-
 Spain's Bonmati 'grateful' for Euros bid after meningitis scare
Spain's Bonmati 'grateful' for Euros bid after meningitis scare
-
'Benign' vein issue behind Trump's swollen legs: White House

-
 Afghan data breach unmasked UK spies, special forces: reports
Afghan data breach unmasked UK spies, special forces: reports
-
US health experts reassess hormone replacement therapy risks

-
 France court orders release of Lebanese militant after 40 years in jail
France court orders release of Lebanese militant after 40 years in jail
-
Goodbye 'Downton Abbey' auction and UK exhibition announced

-
 Soaked Scheffler battles elements to make solid British Open start
Soaked Scheffler battles elements to make solid British Open start
-
Ons Jabeur announces break from tennis 'to rediscover joy of living'

-
 UK, Germany vow to tackle people smuggling gangs
UK, Germany vow to tackle people smuggling gangs
-
Zuckerberg settles lawsuit over Cambridge Analytica scandal

-
 Global markets rise as Trump weighs future of Fed boss
Global markets rise as Trump weighs future of Fed boss
-
Syria troops quit Druze heartland after violence leaves over 500 dead

-
 TikTok Germany moderators raise alarm over layoff plans
TikTok Germany moderators raise alarm over layoff plans
-
Pogacar retakes Tour de France lead in crushing mountain win

-
 Women's marathon world record holder Chepngetich suspended for doping suspicions
Women's marathon world record holder Chepngetich suspended for doping suspicions
-
EU readies retaliatory list targeting US services

-
 'Back in love': MotoGP champion Martin stays with Aprilia
'Back in love': MotoGP champion Martin stays with Aprilia
-
Israeli strike on Gaza's only Catholic church kills three
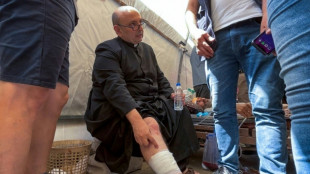
-
 'I'm not an old guy': Usyk says age won't matter in Dubois bout
'I'm not an old guy': Usyk says age won't matter in Dubois bout
-
Fan energy key for Swiss in Euros clash with Spain, says Maritz

-
 'Like a dream': Druze reunited across Golan Heights buffer zone
'Like a dream': Druze reunited across Golan Heights buffer zone
-
US health experts to reassess hormone replacement therapy risks

-
 Scheffler makes bright British Open start before McIlroy takes centre stage
Scheffler makes bright British Open start before McIlroy takes centre stage
-
El Salvador rights group says forced into exile by Bukele crackdown

-
 Shock and sadness as Tomorrowland music festival opens after fire
Shock and sadness as Tomorrowland music festival opens after fire
-
Napoli sign Dutch international forward Lang

-
 Westwood rolls back years on British Open return
Westwood rolls back years on British Open return
-
UK to lower voting age to 16 in general elections

-
 Sri Lanka returns orphaned elephants to the jungle
Sri Lanka returns orphaned elephants to the jungle
-
Russian deputies back fines for clicking on 'extremist' content


US authorizes Juul to market vaping products
Juul Labs said Thursday that the US Food and Drug Administration had officially authorized the e-cigarette maker to market its vaping system and refill capsules in the United States.
Juul won the new marketing granting orders (MGO) after submitting more than 110 scientific studies to the agency, the company said in a statement.
The decision means Juul can continue to sell products that have been on the US market but in regulatory limbo following earlier actions by the FDA.
"Following rigorous evaluation of the data, FDA decided that an MGO for the Juul System was 'appropriate for the protection of public health' –- the standard required by statute for authorization," Juul said.
Juul has argued that its vaping products provide a public health benefit by shifting smokers away from combustible tobacco products closely linked to deadly illnesses.
But the company has been criticized for its marketing practices, agreeing to pay $438.5 million in a 2022 settlement with 34 US states to resolve accusations of marketing to underage smokers.
The FDA's move allows Juul to sell five products in all: the Juul device and capsules for the "Virginia Tobacco" and menthol flavor, each in versions with three or five percent nicotine concentration, said a spokesperson for the US Department of Health and Human Services.
The products needed to meet the standard set under a 2009 smoking prevention law showing that the benefits of switching to a potentially less harmful product is "sufficient to outweigh the risks of the product," including to anyone not using tobacco products, the spokesperson said.
The applicant submitted data "demonstrating high rates of adults completely switching from cigarettes to either the tobacco- or menthol-flavored Juul products."
But the agency's determination "does not mean these tobacco products are safe, nor are they 'FDA approved,'" said the spokesperson, who added that the agency will continue to monitor Juul's compliance with youth marketing restrictions.
Juul survived a difficult stretch after the FDA ordered it to halt sales in June 2022 because of health questions, although the decision was adminsistratively suspended by the same agency shortly thereafter.
In June 2024, the FDA formally rescinded its June 2022 order, shifting the matter back into "pending" status while a substantive review continued.
The FDA's action on Thursday "confirms that these products are now authorized to remain on the market," said a Juul spokesman.
A.Mahlangu--AMWN