-
 Australia's Head backs struggling opening partner Weatherald
Australia's Head backs struggling opening partner Weatherald
-
'Make emitters responsible': Thailand's clean air activists

-
 Zelensky looks to close out Ukraine peace deal at Trump meet
Zelensky looks to close out Ukraine peace deal at Trump meet
-
MCG curator in 'state of shock' after Ashes Test carnage

-
 Texans edge Chargers to reach NFL playoffs
Texans edge Chargers to reach NFL playoffs
-
Osimhen and Mane score as Nigeria win to qualify, Senegal draw

-
 Osimhen stars as Nigeria survive Tunisia rally to reach second round
Osimhen stars as Nigeria survive Tunisia rally to reach second round
-
How Myanmar's junta-run vote works, and why it might not

-
 Watkins wants to sicken Arsenal-supporting family
Watkins wants to sicken Arsenal-supporting family
-
Arsenal hold off surging Man City, Villa as Wirtz ends drought

-
 Late penalty miss denies Uganda AFCON win against Tanzania
Late penalty miss denies Uganda AFCON win against Tanzania
-
Watkins stretches Villa's winning streak at Chelsea

-
 Zelensky stops in Canada en route to US as Russia pummels Ukraine
Zelensky stops in Canada en route to US as Russia pummels Ukraine
-
Arteta salutes injury-hit Arsenal's survival spirit

-
 Wirtz scores first Liverpool goal as Anfield remembers Jota
Wirtz scores first Liverpool goal as Anfield remembers Jota
-
Mane rescues AFCON draw for Senegal against DR Congo

-
 Arsenal hold off surging Man City, Wirtz breaks Liverpool duck
Arsenal hold off surging Man City, Wirtz breaks Liverpool duck
-
Arsenal ignore injury woes to retain top spot with win over Brighton
-
 Sealed with a kiss: Guardiola revels in Cherki starring role
Sealed with a kiss: Guardiola revels in Cherki starring role
-
UK launches paid military gap-year scheme amid recruitment struggles

-
 Jota's children join tributes as Liverpool, Wolves pay respects
Jota's children join tributes as Liverpool, Wolves pay respects
-
'Tired' Inoue beats Picasso by unanimous decision to end gruelling year

-
 Thailand and Cambodia declare truce after weeks of clashes
Thailand and Cambodia declare truce after weeks of clashes
-
Netanyahu to meet Trump in US on Monday

-
 US strikes targeted IS militants, Lakurawa jihadists, Nigeria says
US strikes targeted IS militants, Lakurawa jihadists, Nigeria says
-
Cherki stars in Man City win at Forest

-
 Schwarz records maiden super-G success, Odermatt fourth
Schwarz records maiden super-G success, Odermatt fourth
-
Russia pummels Kyiv ahead of Zelensky's US visit

-
 Smith laments lack of runs after first Ashes home Test loss for 15 years
Smith laments lack of runs after first Ashes home Test loss for 15 years
-
Russian barrage on Kyiv kills one, leaves hundreds of thousands without power

-
 Stokes, Smith agree two-day Tests not a good look after MCG carnage
Stokes, Smith agree two-day Tests not a good look after MCG carnage
-
Stokes hails under-fire England's courage in 'really special' Test win

-
 What they said as England win 4th Ashes Test - reaction
What they said as England win 4th Ashes Test - reaction
-
Hong Kongers bid farewell to 'king of umbrellas'

-
 England snap 15-year losing streak to win chaotic 4th Ashes Test
England snap 15-year losing streak to win chaotic 4th Ashes Test
-
Thailand and Cambodia agree to 'immediate' ceasefire

-
 Closing 10-0 run lifts Bulls over 76ers while Pistons fall
Closing 10-0 run lifts Bulls over 76ers while Pistons fall
-
England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test

-
 Somalia, African nations denounce Israeli recognition of Somaliland
Somalia, African nations denounce Israeli recognition of Somaliland
-
England need 175 to win chaotic 4th Ashes Test

-
 Cricket Australia boss says short Tests 'bad for business' after MCG carnage
Cricket Australia boss says short Tests 'bad for business' after MCG carnage
-
Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan

-
 Six Australia wickets fall as England fight back in 4th Ashes Test
Six Australia wickets fall as England fight back in 4th Ashes Test
-
New to The Street Show #710 Airs Tonight at 6:30 PM EST on Bloomberg Television

-
 Dental Implant Financing and Insurance Options in Georgetown, TX
Dental Implant Financing and Insurance Options in Georgetown, TX
-
Man Utd made to 'suffer' for Newcastle win, says Amorim

-
 Morocco made to wait for Cup of Nations knockout place after Egypt advance
Morocco made to wait for Cup of Nations knockout place after Egypt advance
-
Key NFL week has playoff spots, byes and seeds at stake

-
 Morocco forced to wait for AFCON knockout place after Mali draw
Morocco forced to wait for AFCON knockout place after Mali draw
-
Dorgu delivers winner for depleted Man Utd against Newcastle


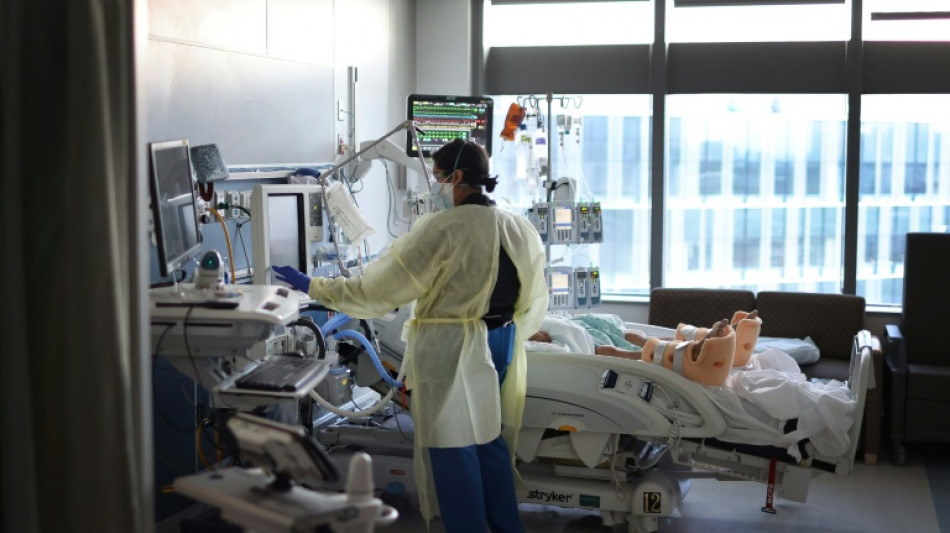
Trial of new Covid treatment yields encouraging results: study
A single-injection antiviral treatment for newly-infected Covid-19 patients reduced the risk of hospitalization by half in a large-scale clinical trial, according to a study published on Wednesday.
Stanford University professor Jeffrey Glenn, coauthor of the study published in the New England Journal of Medicine (NEJM), said the new drug "showed profound benefits for vaccinated and unvaccinated people alike."
While the number of Americans dying daily of coronavirus has fallen to around 500, treatments for the disease remain limited. One of the most common, Paxlovid made by Pfizer, involves taking 30 pills over five days.
The new treatment involves a single dose of pegylated lambda-interferon, a synthetic version of a naturally occurring protein that infected cells secrete to defend against viral infection.
"What it does is it binds receptors on the surfaces of cells that activate our own antiviral defense mechanisms," said Glenn, a professor of medicine, microbiology and immunology who heads the Stanford Biosecurity and Pandemic Preparedness Initiative.
"So if a virus has infected the cell it will turn on processes that aim to destroy the virus's replication," he said. "It will also send signals to neighboring cells to warn them viruses are on their way and get ready to defend yourself."
Receptors for interferon lambda are primarily in the linings of the lungs, airways and intestine -- the main places Covid strikes.
"We're turning on these antiviral mechanisms in the cells, the lung, where the infection is happening," Glenn said.
The Phase 3 trial of the drug, conducted between June 2021 and February 2022, involved nearly 2,000 patients with Covid symptoms in Brazil and Canada, about 85 percent of whom had been vaccinated.
A total of 931 newly-infected Covid patients were given a single injection of interferon lambda while 1,018 participants were given a placebo.
The risk of Covid-19–related hospitalization or death from any cause was 47 percent lower in the interferon group than in the placebo group, according to the researchers.
Twenty-five of the 931 people who received the injection within seven days of exhibiting Covid symptoms were hospitalized compared with 57 of the 1,018 who received the placebo.
Vaccinated patients treated with interferon lambda experienced a 51 percent reduction in hospitalization relative to the placebo group.
There was an 89 percent reduction in hospitalization among unvaccinated patients treated within the first three days of the onset of Covid symptoms compared with the placebo group.
- Developed for hepatitis D -
Glenn said interferon lambda proved effective against all Covid variants tested, including Omicron, and side effects in the group receiving the injections were no greater than among the placebo recipients.
Glenn is the founder of a small biotechnology company called Eiger Biopharmaceuticals that acquired interferon lambda to develop drugs for hepatitis delta virus.
"When Covid came, I said this would be the perfect drug for Covid," said Glenn, who left the Palo Alto-based company but remains on the board of directors and is an equity holder.
Eiger sought an emergency use authorization for interferon lambda from the US Federal Drug Administration (FDA) for Covid treatment last year but it was not granted.
That was "very frustrating," Glenn said, but he was hopeful publication of the study in the NEJM "will help encourage regulators here and around the world to find a way to get lambda into patients as soon as possible."
P.Costa--AMWN



