
-
 New NBA dunk contest champ assured and shooting stars return
New NBA dunk contest champ assured and shooting stars return
-
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games

-
 Takaichi tipped for big win as Japan votes
Takaichi tipped for big win as Japan votes
-
Lens return top of Ligue 1 with win over Rennes

-
 Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
-
Demonstrators in Berlin call for fall of Iran's Islamic republic

-
 'Free the mountains!": clashes at Milan protest over Winter Olympics
'Free the mountains!": clashes at Milan protest over Winter Olympics
-
Townsend accepts pressure will mount on him after Italy defeat

-
 BMW iX3 new style and design
BMW iX3 new style and design
-
Suryakumar's 84 leads India to opening win over USA in T20 World Cup

-
 Lollobrigida skates to first Italian gold of Milan-Cortina Games
Lollobrigida skates to first Italian gold of Milan-Cortina Games
-
Barca beat Mallorca to extend Liga lead

-
 Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
-
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga

-
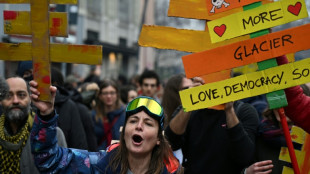 'Free the mountains!": protest in Milan over Winter Olympics
'Free the mountains!": protest in Milan over Winter Olympics
-
Gyokeres double helps Arsenal stretch Premier League lead

-
 New Skoda Epiq: modern with range
New Skoda Epiq: modern with range
-
Six Nations misery for Townsend as Italy beat sorry Scotland

-
 Spain, Portugal face fresh storms, torrential rain
Spain, Portugal face fresh storms, torrential rain
-
Opinions of Zuckerberg hang over social media addiction trial jury selection

-
 Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
-
Norway's Ruud tops Olympic men's freeski slopestyle qualifying

-
 Czech qualifier Bejlek claims first title in Abu Dhabi
Czech qualifier Bejlek claims first title in Abu Dhabi
-
French duo reach Shanghai, completing year-and-a-half walk

-
 Australian snowboarder James eyes elusive Olympic gold
Australian snowboarder James eyes elusive Olympic gold
-
Sequins and snow: Eva Adamczykova makes Olympic return

-
 Vonn set for Olympic medal bid after successful downhill training
Vonn set for Olympic medal bid after successful downhill training
-
Shepherd takes hat-trick as West Indies beat Scotland in T20 World Cup

-
 Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
-
Swiss racer Von Allmen wins first gold of Winter Olympics

-
 'Wake up': Mum sparks comeback after scare for freeski star Gu
'Wake up': Mum sparks comeback after scare for freeski star Gu
-
Von Allmen wins men's Olympic downhill gold, first of Games

-
 First medals up for grabs at Winter Olympics
First medals up for grabs at Winter Olympics
-
Afghanistan captain Khan harbours dream of playing in Kabul

-
 Lindsey Vonn completes second Winter Olympics downhill training run
Lindsey Vonn completes second Winter Olympics downhill training run
-
Freeski star Gu survives major scare in Olympic slopestyle

-
 Iran FM looks to more nuclear talks, but warns US
Iran FM looks to more nuclear talks, but warns US
-
Hetmyer's six-hitting steers West Indies to 182-5 against Scotland

-
 After boos for Vance, IOC says it hopes for 'fair play'
After boos for Vance, IOC says it hopes for 'fair play'
-
Thousands gather as Pakistan buries victims of mosque suicide attack

-
 Lindsey Vonn completes second downhill training session
Lindsey Vonn completes second downhill training session
-
US pressing Ukraine and Russia to end war by June, Zelensky says

-
 Faheem blitz sees Pakistan avoid Netherlands shock at T20 World Cup
Faheem blitz sees Pakistan avoid Netherlands shock at T20 World Cup
-
Takaichi talks tough on immigration on eve of vote

-
 England's Salt passed fit for T20 World Cup opener
England's Salt passed fit for T20 World Cup opener
-
Spain, Portugal brace for fresh storm after flood deaths

-
 Pakistan bowl out Netherlands for 147 in T20 World Cup opener
Pakistan bowl out Netherlands for 147 in T20 World Cup opener
-
Pushed to margins, women vanish from Bangladesh's political arena

-
 Crypto firm accidentally sends $40 bn in bitcoin to users
Crypto firm accidentally sends $40 bn in bitcoin to users
-
Pistons end Knicks' NBA winning streak, Celtics edge Heat


US authorizes first condom for use in anal sex
The US Food and Drug Administration (FDA) on Wednesday authorized the first condom for use during anal intercourse, in what was hailed as a victory for sexual health by experts.
Although people already use condoms for anal sex -- as is recommended by health agencies including the Centers for Disease Control and Prevention (CDC) -- regulators across the world had only previously allowed companies to officially market their products as "safe and effective" for vaginal use.
Sexual health advocates considered this an unmet public health need since unprotected anal intercourse carries the greatest risk of HIV transmission via sexual exposure, with one study finding that 69 percent of men who have sex with men would use condoms more frequently if they were FDA-indicated.
Wednesday's authorization of Global Protection Corp's One Male Condom follows a clinical trial involving more than 500 people, carried out by Emory University.
"The FDA's authorization of a condom that is specifically indicated, evaluated and labeled for anal intercourse may improve the likelihood of condom use during anal intercourse," said agency scientist Courtney Lias in a statement.
The condom is also indicated to prevent sexually transmitted infections -- and as a contraceptive -- during vaginal sex.
"We want people to have lots of sex -- but we also want them to be empowered and informed," said Davin Wedel, president of Global Protection Corp, which makes the condom brand that is available in 54 sizes, and incorporates a paper template to help each user find the right size.
The clinical trial involved 252 men who have sex with men and 252 men who have sex with women, aged between 18 and 54.
The FDA had said it would accept a five percent failure rate, which previous trials had failed to accomplish. The limit was easily surpassed in the new study, with the failure rate 0.68 percent for anal and 1.89 percent for vaginal intercourse.
The researchers behind the study, which was published in The Lancet's eClinicalMedicine, said one of the reasons the trial succeeded where others failed in the past was likely due to the provision of lubricant and inclusion of instructions on how to use the product.
Lubricant reduces friction, which in turn causes condom failure from slippage and breakage.
Another reason could be that participants were asked to keep mobile phone-based daily diaries, whereas past trials had asked volunteers to recall failure events up to several months later.
Monica Gandhi, an infectious disease doctor and medical director of an HIV clinic in San Francisco, welcomed the finding.
"The important thing about condoms is they don't just prevent HIV, but they prevent gonorrhea, chlamydia and syphilis," she told AFP, adding it was surprising that such an authorization had taken so long to achieve.
In its statement, the FDA said the green light could pave the way for more makers to apply for similar authorization if they show equivalent results.
P.Mathewson--AMWN


