
-
 How Myanmar's junta-run vote works, and why it might not
How Myanmar's junta-run vote works, and why it might not
-
Watkins wants to sicken Arsenal-supporting family

-
 Arsenal hold off surging Man City, Villa as Wirtz ends drought
Arsenal hold off surging Man City, Villa as Wirtz ends drought
-
Late penalty miss denies Uganda AFCON win against Tanzania

-
 Watkins stretches Villa's winning streak at Chelsea
Watkins stretches Villa's winning streak at Chelsea
-
Zelensky stops in Canada en route to US as Russia pummels Ukraine

-
 Arteta salutes injury-hit Arsenal's survival spirit
Arteta salutes injury-hit Arsenal's survival spirit
-
Wirtz scores first Liverpool goal as Anfield remembers Jota

-
 Mane rescues AFCON draw for Senegal against DR Congo
Mane rescues AFCON draw for Senegal against DR Congo
-
Arsenal hold off surging Man City, Wirtz breaks Liverpool duck

-
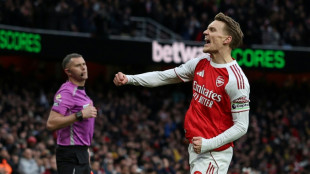 Arsenal ignore injury woes to retain top spot with win over Brighton
Arsenal ignore injury woes to retain top spot with win over Brighton
-
Sealed with a kiss: Guardiola revels in Cherki starring role

-
 UK launches paid military gap-year scheme amid recruitment struggles
UK launches paid military gap-year scheme amid recruitment struggles
-
Jota's children join tributes as Liverpool, Wolves pay respects

-
 'Tired' Inoue beats Picasso by unanimous decision to end gruelling year
'Tired' Inoue beats Picasso by unanimous decision to end gruelling year
-
Thailand and Cambodia declare truce after weeks of clashes

-
 Netanyahu to meet Trump in US on Monday
Netanyahu to meet Trump in US on Monday
-
US strikes targeted IS militants, Lakurawa jihadists, Nigeria says

-
 Cherki stars in Man City win at Forest
Cherki stars in Man City win at Forest
-
Schwarz records maiden super-G success, Odermatt fourth

-
 Russia pummels Kyiv ahead of Zelensky's US visit
Russia pummels Kyiv ahead of Zelensky's US visit
-
Smith laments lack of runs after first Ashes home Test loss for 15 years

-
 Russian barrage on Kyiv kills one, leaves hundreds of thousands without power
Russian barrage on Kyiv kills one, leaves hundreds of thousands without power
-
Stokes, Smith agree two-day Tests not a good look after MCG carnage

-
 Stokes hails under-fire England's courage in 'really special' Test win
Stokes hails under-fire England's courage in 'really special' Test win
-
What they said as England win 4th Ashes Test - reaction

-
 Hong Kongers bid farewell to 'king of umbrellas'
Hong Kongers bid farewell to 'king of umbrellas'
-
England snap 15-year losing streak to win chaotic 4th Ashes Test

-
 Thailand and Cambodia agree to 'immediate' ceasefire
Thailand and Cambodia agree to 'immediate' ceasefire
-
Closing 10-0 run lifts Bulls over 76ers while Pistons fall

-
 England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test
England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test
-
Somalia, African nations denounce Israeli recognition of Somaliland

-
 England need 175 to win chaotic 4th Ashes Test
England need 175 to win chaotic 4th Ashes Test
-
Cricket Australia boss says short Tests 'bad for business' after MCG carnage

-
 Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan
Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan
-
Six Australia wickets fall as England fight back in 4th Ashes Test

-
 Dental Implant Financing and Insurance Options in Georgetown, TX
Dental Implant Financing and Insurance Options in Georgetown, TX
-
Man Utd made to 'suffer' for Newcastle win, says Amorim

-
 Morocco made to wait for Cup of Nations knockout place after Egypt advance
Morocco made to wait for Cup of Nations knockout place after Egypt advance
-
Key NFL week has playoff spots, byes and seeds at stake

-
 Morocco forced to wait for AFCON knockout place after Mali draw
Morocco forced to wait for AFCON knockout place after Mali draw
-
Dorgu delivers winner for depleted Man Utd against Newcastle

-
 US stocks edge lower from records as precious metals surge
US stocks edge lower from records as precious metals surge
-
Somalia denounces Israeli recognition of Somaliland

-
 The Cure guitarist and keyboard player Perry Bamonte dies aged 65
The Cure guitarist and keyboard player Perry Bamonte dies aged 65
-
Draper to miss Australian Open

-
 Police arrest suspect after man stabs 3 women in Paris metro
Police arrest suspect after man stabs 3 women in Paris metro
-
Former Montpellier coach Gasset dies at 72

-
 Trump's Christmas gospel: bombs, blessings and blame
Trump's Christmas gospel: bombs, blessings and blame
-
Russia lashes out at Zelensky ahead of new Trump meeting on Ukraine plan


US becomes first country to approve RSV vaccine
The United States on Wednesday approved the world's first vaccine for the Respiratory Syncytial Virus (RSV), the culmination of a decades-long hunt to protect vulnerable people from the common illness.
Drugmaker GSK's Arexvy was green-lighted for adults aged 60 and older, with similar shots from other makers including Pfizer and Moderna expected to follow soon.
"Today's approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening," said senior US Food and Drug Administration (FDA) official Peter Marks in a statement.
The decision "marks a turning point in our effort to reduce the significant burden of RSV," added Tony Wood, GSK's chief scientific officer.
RSV is a common virus that normally causes mild, cold-like symptoms, but can be serious for infants and the elderly, as well as those with weak immune systems and underlying conditions.
In severe cases it can cause pneumonia and bronchiolitis, an inflammation of the small airways deep inside the lungs.
According to the US Centers for Disease Control and Prevention, RSV leads to approximately 60,000 to 120,000 hospitalizations and 6,000 to 10,000 deaths among adults 65 years of age and older.
Awareness of the disease has increased in recent years, in part because of the strain it has placed on hospital systems over the last two winters.
Rates of RSV and flu fell during Covid-19 lockdowns, but surged when restrictions were lifted, with young children hit hard.
Pharmaceutical companies have been chasing an RSV vaccine for years. Given recent successful breakthroughs in the sector, analysts predict the market could be worth over $10 billion in the next decade, according to reports.
- More vaccines on way -
GSK's vaccine contains a "subunit" or part of the virus to help train the immune system should it encounter the real thing.
It was approved based on a study of 25,000 people aged 60 and older that showed a single dose was 83 percent effective against disease caused by RSV, and more than 94 percent effective against severe disease.
Researchers will continue to follow volunteers in the study to assess the duration of protection as well as the safety and efficacy of more doses.
The most common side effects included injection site pain, fatigue, muscle pain, headaches and joint stiffness.
An irregular heartbeat was a less common side effect, occurring in 10 participants who received Arexvy and four participants who received placebo.
Safety issues were also found in two other studies of the drug involving approximately 2,500 people aged 60 and up. In one of these studies, two volunteers developed a rare type of inflammation that affects the brain and spinal cord, and one of them died.
In the other study, one participant developed Guillain-Barre syndrome, in which the immune system damages nerve cells, causing muscle weakness and sometimes paralysis.
GSK's Arexvy was recommended for approval last week by the European Union's drug watchdog, the European Medicines Agency, whose positive opinions are normally formally followed by approval from the European Commission.
Pfizer has said that it expects a decision from the FDA in May for its own RSV vaccine, also for those over 60 years old.
In January, Moderna said it hopes its RSV vaccine will be approved and available in time for the Northern Hemisphere's winter later this year.
Several other companies are also developing RSV vaccines.
Last year, the EU approved a preventative antibody treatment against RSV, developed by British-Swedish pharmaceutical firm AstraZeneca and France's Sanofi, which confers temporary protection.
Ch.Havering--AMWN



