
-
 Wirtz scores first Liverpool goal as Anfield remembers Jota
Wirtz scores first Liverpool goal as Anfield remembers Jota
-
Mane rescues AFCON draw for Senegal against DR Congo

-
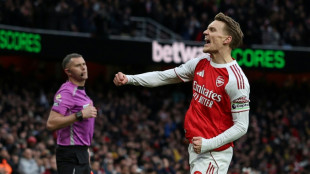
 Arsenal hold off surging Man City, Wirtz breaks Liverpool duck
Arsenal hold off surging Man City, Wirtz breaks Liverpool duck
-
Arsenal ignore injury woes to retain top spot with win over Brighton
-
 Sealed with a kiss: Guardiola revels in Cherki starring role
Sealed with a kiss: Guardiola revels in Cherki starring role
-
UK launches paid military gap-year scheme amid recruitment struggles

-
 Jota's children join tributes as Liverpool, Wolves pay respects
Jota's children join tributes as Liverpool, Wolves pay respects
-
'Tired' Inoue beats Picasso by unanimous decision to end gruelling year

-
 Thailand and Cambodia declare truce after weeks of clashes
Thailand and Cambodia declare truce after weeks of clashes
-
Netanyahu to meet Trump in US on Monday

-
 US strikes targeted IS militants, Lakurawa jihadists, Nigeria says
US strikes targeted IS militants, Lakurawa jihadists, Nigeria says
-
Cherki stars in Man City win at Forest

-
 Schwarz records maiden super-G success, Odermatt fourth
Schwarz records maiden super-G success, Odermatt fourth
-
Russia pummels Kyiv ahead of Zelensky's US visit

-
 Smith laments lack of runs after first Ashes home Test loss for 15 years
Smith laments lack of runs after first Ashes home Test loss for 15 years
-
Russian barrage on Kyiv kills one, leaves hundreds of thousands without power

-
 Stokes, Smith agree two-day Tests not a good look after MCG carnage
Stokes, Smith agree two-day Tests not a good look after MCG carnage
-
Stokes hails under-fire England's courage in 'really special' Test win

-
 What they said as England win 4th Ashes Test - reaction
What they said as England win 4th Ashes Test - reaction
-
Hong Kongers bid farewell to 'king of umbrellas'

-
 England snap 15-year losing streak to win chaotic 4th Ashes Test
England snap 15-year losing streak to win chaotic 4th Ashes Test
-
Thailand and Cambodia agree to 'immediate' ceasefire

-
 Closing 10-0 run lifts Bulls over 76ers while Pistons fall
Closing 10-0 run lifts Bulls over 76ers while Pistons fall
-
England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test

-
 Somalia, African nations denounce Israeli recognition of Somaliland
Somalia, African nations denounce Israeli recognition of Somaliland
-
England need 175 to win chaotic 4th Ashes Test

-
 Cricket Australia boss says short Tests 'bad for business' after MCG carnage
Cricket Australia boss says short Tests 'bad for business' after MCG carnage
-
Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan

-
 Six Australia wickets fall as England fight back in 4th Ashes Test
Six Australia wickets fall as England fight back in 4th Ashes Test
-
Dental Implant Financing and Insurance Options in Georgetown, TX

-
 Man Utd made to 'suffer' for Newcastle win, says Amorim
Man Utd made to 'suffer' for Newcastle win, says Amorim
-
Morocco made to wait for Cup of Nations knockout place after Egypt advance

-
 Key NFL week has playoff spots, byes and seeds at stake
Key NFL week has playoff spots, byes and seeds at stake
-
Morocco forced to wait for AFCON knockout place after Mali draw

-
 Dorgu delivers winner for depleted Man Utd against Newcastle
Dorgu delivers winner for depleted Man Utd against Newcastle
-
US stocks edge lower from records as precious metals surge

-
 Somalia denounces Israeli recognition of Somaliland
Somalia denounces Israeli recognition of Somaliland
-
The Cure guitarist and keyboard player Perry Bamonte dies aged 65

-
 Draper to miss Australian Open
Draper to miss Australian Open
-
Police arrest suspect after man stabs 3 women in Paris metro

-
 Former Montpellier coach Gasset dies at 72
Former Montpellier coach Gasset dies at 72
-
Trump's Christmas gospel: bombs, blessings and blame

-
 Russia lashes out at Zelensky ahead of new Trump meeting on Ukraine plan
Russia lashes out at Zelensky ahead of new Trump meeting on Ukraine plan
-
Salah helps Egypt beat South Africa and book last-16 place

-
 Australia's Ikitau facing lengthy lay-off after shoulder injury
Australia's Ikitau facing lengthy lay-off after shoulder injury
-
Another 1,100 refugees cross into Mauritania from Mali: UN

-
 Guardiola proud of Man City players' response to weighty issues
Guardiola proud of Man City players' response to weighty issues
-
Deadly blast hits mosque in Alawite area of Syria's Homs

-
 The Jukebox Man on song as Redknapp records 'dream' King George win
The Jukebox Man on song as Redknapp records 'dream' King George win
-
Liverpool boss Slot says Ekitike reaping rewards for greater physicality


US makes new Alzheimer's drug more widely accessible
The US drug regulator gave full approval to a new Alzheimer's medicine on Thursday, a move that makes it more widely available to the public through government-run health insurance for the elderly.
Leqembi, developed jointly by Japan's Eisai and Biogen of the United States, was shown in a clinical trial to modestly reduce cognitive decline among patients in the early stages of the disease.
But the study also raised concerns about side effects including brain bleeds and swelling.
Leqembi was initially granted "accelerated approval" by the Food and Drug Administration in January, which meant it was not broadly covered by the government-run Medicare program for people aged 65 and older.
Thursday's decision, which follows further study of the drug, means Medicare will now defray a large portion of treatment, initially listed by the makers at $26,500 per year.
"This confirmatory study verified that it is a safe and effective treatment for patients with Alzheimer's disease," senior FDA official Teresa Buracchio said in a statement.
Chiquita Brooks-LaSure, administrator of the agency that runs Medicare, added: "This is welcome news for the millions of people in this country and their families who are affected by this debilitating disease."
But people covered by Medicare will still need to meet 20 percent of the cost, or thousands of dollars, themselves.
Approximately 6.5 million Americans suffer from Alzheimer's, which is characterized by memory loss and declining mental acuity.
Leqembi, also known as lecanemab, is an antibody treatment that is injected into the brain every two weeks and works by reducing amyloid beta, a protein that builds into plaques and causes brain cells to die, as well as brain shrinkage.
The FDA's decision was welcomed by patient groups.
"This treatment, while not a cure, can give people in the early stages of Alzheimer's more time to maintain their independence and do the things they love," said Joanne Pike, Alzheimer's Association president and CEO.
"This gives people more months of recognizing their spouse, children and grandchildren."
Leqembi was the second Alzheimer's drug developed by Eisai and Biogen to receive approval. The first, Aduhelm, was approved in 2021 but the decision was highly controversial as the data about its efficacy was inconsistent.
In May, US drugmaker Eli Lilly announced its drug donanemab also significantly slowed cognitive decline associated with Alzheimer's, and would soon seek worldwide regulatory approval.
Alzheimer's disease accounts for 60 to 80 percent of dementia, according to the Alzheimer's Association. It progressively destroys thinking and memory, eventually robbing people of the ability to carry out the simplest of tasks.
D.Kaufman--AMWN



