-
 Seifert powers New Zealand to their record T20 World Cup chase
Seifert powers New Zealand to their record T20 World Cup chase
-
Naib's fifty lifts Afghanistan to 182-6 against New Zealand

-
 Paul Thomas Anderson wins top director prize for 'One Battle After Another'
Paul Thomas Anderson wins top director prize for 'One Battle After Another'
-
De Beers sale drags in diamond doldrums

-
 NFL embraces fashion as league seeks new audiences
NFL embraces fashion as league seeks new audiences
-
What's at stake for Indian agriculture in Trump's trade deal?

-
 Real Madrid can wait - Siraj's dream night after late T20 call-up
Real Madrid can wait - Siraj's dream night after late T20 call-up
-
Castle's monster night fuels Spurs, Rockets rally to beat Thunder
-
 Japan votes in snow-hit snap polls as Takaichi eyes strong mandate
Japan votes in snow-hit snap polls as Takaichi eyes strong mandate
-
Pakistan's capital picks concrete over trees, angering residents

-
 Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo
Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo
-
Neglected killer: kala-azar disease surges in Kenya

-
 Super Bowl set for Patriots-Seahawks showdown as politics swirl
Super Bowl set for Patriots-Seahawks showdown as politics swirl
-
Sengun shines as Rockets rally to beat NBA champion Thunder

-
 Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back
Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back
-
Washington Post CEO out after sweeping job cuts

-
 Haiti's transitional council hands power to PM
Haiti's transitional council hands power to PM
-
N. Korea to hold party congress in February, first since 2021

-
 Thailand votes after three leaders in two years
Thailand votes after three leaders in two years
-
Swiss joy as Von Allmen wins first gold of Winter Olympics

-
 George backs England to 'kick on' after Six Nations rout of Wales
George backs England to 'kick on' after Six Nations rout of Wales
-
Malinin upstaged as Japan keep pressure on USA in skating team event

-
 Vail's golden comets Vonn and Shiffrin inspire those who follow
Vail's golden comets Vonn and Shiffrin inspire those who follow
-
Veteran French politician loses culture post over Epstein links

-
 Japan's Kimura wins Olympic snowboard big air gold
Japan's Kimura wins Olympic snowboard big air gold
-
Arteta backs confident Gyokeres to hit 'highest level'

-
 Hojlund the hero as Napoli snatch late win at Genoa
Hojlund the hero as Napoli snatch late win at Genoa
-
England's Arundell 'frustrated' despite hat-trick in Wales romp

-
 Lollobrigida skates to first Italian gold of Winter Olympics on her birthday
Lollobrigida skates to first Italian gold of Winter Olympics on her birthday
-
Arundell hat-trick inspires England thrashing of Wales in Six Nations opener

-
 Chile's climate summit chief to lead plastic pollution treaty talks
Chile's climate summit chief to lead plastic pollution treaty talks
-
Rosenior hails 'unstoppable' Palmer after treble tames Wolves

-
 French ex-minister offers resignation from Paris cultural hub over Epstein links
French ex-minister offers resignation from Paris cultural hub over Epstein links
-
New NBA dunk contest champ assured and shooting stars return

-
 Shiffrin says will use lessons learnt from Beijing flop at 2026 Games
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games
-
Takaichi tipped for big win as Japan votes

-
 Lens return top of Ligue 1 with win over Rennes
Lens return top of Ligue 1 with win over Rennes
-
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow

-
 Demonstrators in Berlin call for fall of Iran's Islamic republic
Demonstrators in Berlin call for fall of Iran's Islamic republic
-
'Free the mountains!": clashes at Milan protest over Winter Olympics

-
 Townsend accepts pressure will mount on him after Italy defeat
Townsend accepts pressure will mount on him after Italy defeat
-
BMW iX3 new style and design

-
 Suryakumar's 84 leads India to opening win over USA in T20 World Cup
Suryakumar's 84 leads India to opening win over USA in T20 World Cup
-
Lollobrigida skates to first Italian gold of Milan-Cortina Games

-
 Barca beat Mallorca to extend Liga lead
Barca beat Mallorca to extend Liga lead
-
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank

-
 Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
-
'Free the mountains!": protest in Milan over Winter Olympics
-
 Gyokeres double helps Arsenal stretch Premier League lead
Gyokeres double helps Arsenal stretch Premier League lead
-
New Skoda Epiq: modern with range


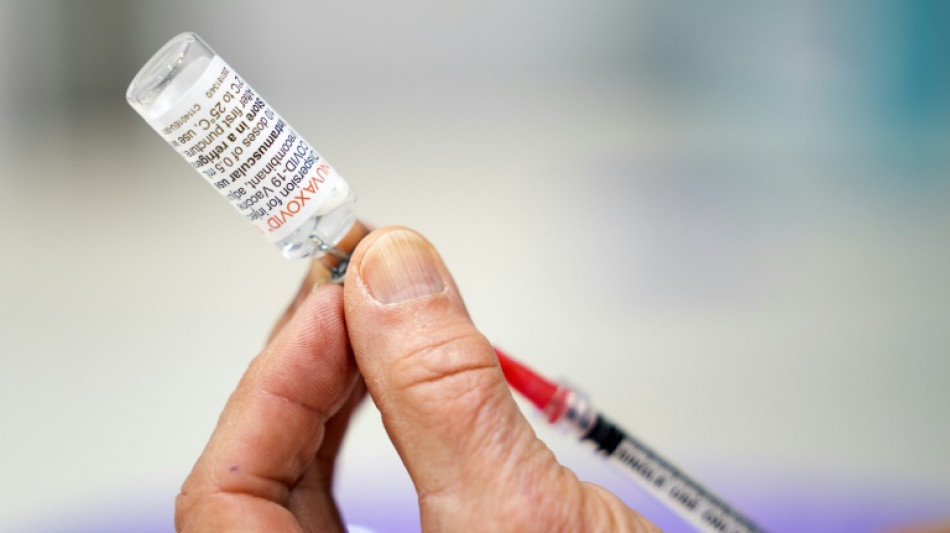
FDA experts weigh authorizing Novavax Covid-19 vaccine in US
A panel of experts convened by the US drug regulator was meeting Tuesday to consider authorizing the Novavax Covid-19 shot, a late runner in the fight against the virus that could nonetheless play a role in overcoming vaccine hesitancy.
Three vaccines are currently approved in the United States: Pfizer and Moderna, which are based on messenger RNA, and Johnson and Johnson, an adenovirus vector vaccine.
But the last of these, the J&J vaccine, was recently restricted in the US after being linked to a rare but serious clotting condition, especially in women of reproductive age.
It is now only recommended for adults who cannot access Pfizer or Moderna for medical or other serious reasons.
The Novavax vaccine was an early frontrunner in the vaccine race, but fell behind after being hit by manufacturing and regulatory delays.
Though the company is American, the US is one of the few major markets where it hasn't yet received authorization -- the EU, UK, Canada, Australia are among many that have already given it the green light.
Officials hope that the shot, which is based on synthetic proteins, could provide an alternative to the mRNA vaccines for people still hesitant.
"We do have a problem with vaccine uptake that is very serious in the United States," Peter Marks, a senior scientist for the Food and Drug administration, said at the start of the meeting.
"And anything we can do to get people more comfortable to be able to accept these potentially life saving medical products, is something that we feel we are compelled to do."
Of the various vaccine technologies, mRNA has been subject to the most misinformation efforts.
Novavax's vaccine was found to be 90 percent effective against symptomatic cases of the disease, in trials conducted before the appearance of the Omicron variant, according to the FDA.
But six cases of myocarditis, an inflammation of the heart muscle, were detected in the group that received the vaccine, against one case in the placebo group, in a trial of around 40,000 people.
Novavax says there is insufficient evidence to establish a causal relationship between the cases of myocarditis and the vaccine.
The FDA voiced concern over the myocarditis link on Friday, sending Novavax shares to drop 20 percent on the New York Stock Exchange. And trading of Novavax stock was halted on Monday pending news from the FDA panel.
Known as a protein subunit vaccine, Novavax is administered in two doses.
It uses a synthetic version of the virus' spike protein to evoke an immune response.
The same technique is used in vaccines against whooping cough, meningococcal meningitis and hepatitis B.
D.Moore--AMWN


