-
 Sarr strikes as Palace end winless run at Brighton
Sarr strikes as Palace end winless run at Brighton
-
Olympic star Ledecka says athletes ignored in debate over future of snowboard event

-
 Auger-Aliassime retains Montpellier Open crown
Auger-Aliassime retains Montpellier Open crown
-
Lindsey Vonn, skiing's iron lady whose Olympic dream ended in tears

-
 Conservative Thai PM claims election victory
Conservative Thai PM claims election victory
-
Kamindu fireworks rescue Sri Lanka to 163-6 against Ireland

-
 UK PM's top aide quits in scandal over Mandelson links to Epstein
UK PM's top aide quits in scandal over Mandelson links to Epstein
-
Reed continues Gulf romp with victory in Qatar

-
 Conservative Thai PM heading for election victory: projections
Conservative Thai PM heading for election victory: projections
-
Heartache for Olympic downhill champion Johnson after Vonn's crash

-
 Takaichi on course for landslide win in Japan election
Takaichi on course for landslide win in Japan election
-
Wales coach Tandy will avoid 'knee-jerk' reaction to crushing England loss

-
 Sanae Takaichi, Japan's triumphant first woman PM
Sanae Takaichi, Japan's triumphant first woman PM
-
England avoid seismic shock by beating Nepal in last-ball thriller

-
 Karl defends Olympic men's parallel giant slalom crown
Karl defends Olympic men's parallel giant slalom crown
-
Colour and caution as banned kite-flying festival returns to Pakistan

-
 England cling on to beat Nepal in last-ball thriller
England cling on to beat Nepal in last-ball thriller
-
UK foreign office to review pay-off to Epstein-linked US envoy

-
 England's Arundell eager to learn from Springbok star Kolbe
England's Arundell eager to learn from Springbok star Kolbe
-
Czech snowboard great Ledecka fails in bid for third straight Olympic gold

-
 Expectation, then stunned silence as Vonn crashes out of Olympics
Expectation, then stunned silence as Vonn crashes out of Olympics
-
Storm-battered Portugal votes in presidential election run-off

-
 Breezy Johnson wins Olympic downhill gold, Vonn crashes out
Breezy Johnson wins Olympic downhill gold, Vonn crashes out
-
Vonn's Olympic dream cut short by downhill crash

-
 French police arrest five over crypto-linked magistrate kidnapping
French police arrest five over crypto-linked magistrate kidnapping
-
Late Jacks flurry propels England to 184-7 against Nepal

-
 Vonn crashes out of Winter Olympics, ending medal dream
Vonn crashes out of Winter Olympics, ending medal dream
-
All-new Ioniq 3 coming in 2026

-
 New Twingo e-tech is at the starting line
New Twingo e-tech is at the starting line
-
New Ypsilon and Ypsilon hf

-
 The Cupra Raval will be launched in 2026
The Cupra Raval will be launched in 2026
-
New id.Polo comes electric

-
 Iran defies US threats to insist on right to enrich uranium
Iran defies US threats to insist on right to enrich uranium
-
Seifert powers New Zealand to their record T20 World Cup chase

-
 Naib's fifty lifts Afghanistan to 182-6 against New Zealand
Naib's fifty lifts Afghanistan to 182-6 against New Zealand
-
Paul Thomas Anderson wins top director prize for 'One Battle After Another'

-
 De Beers sale drags in diamond doldrums
De Beers sale drags in diamond doldrums
-
NFL embraces fashion as league seeks new audiences

-
 What's at stake for Indian agriculture in Trump's trade deal?
What's at stake for Indian agriculture in Trump's trade deal?
-
Real Madrid can wait - Siraj's dream night after late T20 call-up

-
 Castle's monster night fuels Spurs, Rockets rally to beat Thunder
Castle's monster night fuels Spurs, Rockets rally to beat Thunder
-
Japan votes in snow-hit snap polls as Takaichi eyes strong mandate

-
 Pakistan's capital picks concrete over trees, angering residents
Pakistan's capital picks concrete over trees, angering residents
-
Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo

-
 Neglected killer: kala-azar disease surges in Kenya
Neglected killer: kala-azar disease surges in Kenya
-
Super Bowl set for Patriots-Seahawks showdown as politics swirl

-
 Sengun shines as Rockets rally to beat NBA champion Thunder
Sengun shines as Rockets rally to beat NBA champion Thunder
-
Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back

-
 How Dental Implants Can Improve Your Quality of Life in Bonita Springs
How Dental Implants Can Improve Your Quality of Life in Bonita Springs
-
Washington Post CEO out after sweeping job cuts


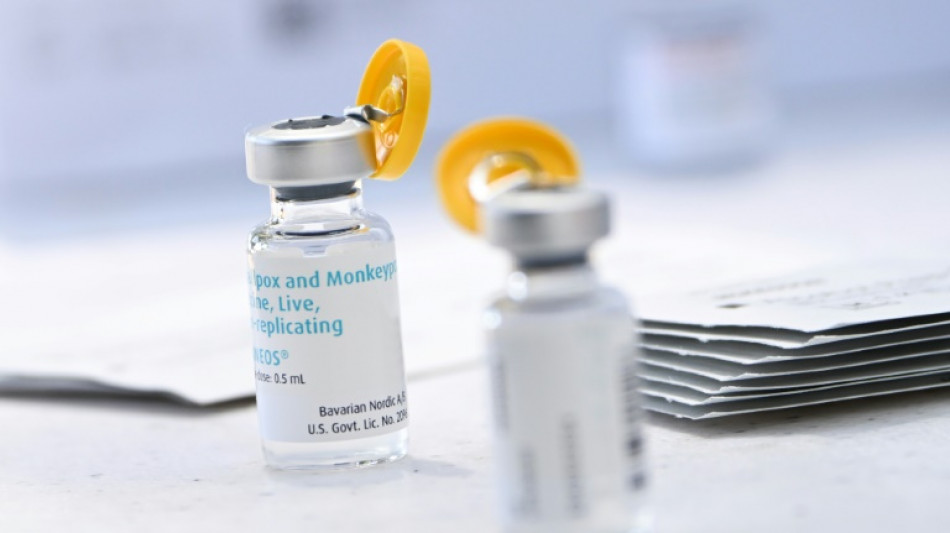
New US strategy to make monkeypox vaccine go further
US health authorities on Tuesday authorized a new procedure for injecting the monkeypox vaccine that should make it possible to inoculate more people with the same amount of the drug, at a time when doses are running short in the country.
The Food and Drug Administration also authorized giving the vaccine to people under the age of 18 who are considered to be at high risk of infection.
For those over 18, health workers will now be able to administer the vaccine differently, via an intradermal injection -- that is, between the upper layers of the skin -- and not with a deeper, subcutaneous injection.
The new method will "increase the total number of doses available for use by up to five-fold," the FDA said in a statement.
Two injections, four weeks apart, will still be necessary.
The FDA said it was drawing on data from a 2015 clinical trial that showed a similar immune response in people given a subcutaneous injection compared to those given a fifth of the dose via an intradermal shot.
At present, some 620,000 doses of the vaccine -- manufactured by Bavarian Nordic, and marketed under the name Jynneos in the United States -- have been distributed across the country.
Another 440,000 additional doses are still to be distributed, which could allow up to 2.2 million injections under the new strategy.
The government has also ordered an additional five million doses, which will start arriving from September and run through 2023, affording the potential to administer 25 million doses.
The decisions came after the FDA issued an emergency use authorization for the vaccine, a move that itself followed the declaration of a public health emergency last week.
For the authorization in minors, the FDA said it had reviewed safety data for the vaccine, as well as data for another vaccine given in children against smallpox.
"We feel very comfortable with the safety of the approach," said Peter Marks of the FDA at a press conference, noting a recent increase in the number of children who have potentially been exposed to infected people.
The United States has registered nearly 9,000 cases of monkeypox, a fifth of them in New York state. The vast majority of cases involve men who have had sex with men.
O.Karlsson--AMWN


