-
 Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
-
'Free the mountains!": protest in Milan over Winter Olympics
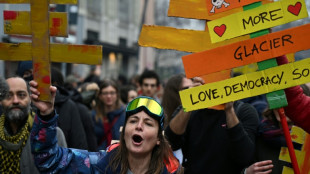
-
 Gyokeres double helps Arsenal stretch Premier League lead
Gyokeres double helps Arsenal stretch Premier League lead
-
Six Nations misery for Townsend as Italy beat sorry Scotland

-
 Spain, Portugal face fresh storms, torrential rain
Spain, Portugal face fresh storms, torrential rain
-
Opinions of Zuckerberg hang over social media addiction trial jury selection

-
 Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official
-
Norway's Ruud tops Olympic men's freeski slopestyle qualifying

-
 Czech qualifier Bejlek claims first title in Abu Dhabi
Czech qualifier Bejlek claims first title in Abu Dhabi
-
French duo reach Shanghai, completing year-and-a-half walk

-
 Australian snowboarder James eyes elusive Olympic gold
Australian snowboarder James eyes elusive Olympic gold
-
Sequins and snow: Eva Adamczykova makes Olympic return

-
 Vonn set for Olympic medal bid after successful downhill training
Vonn set for Olympic medal bid after successful downhill training
-
Shepherd takes hat-trick as West Indies beat Scotland in T20 World Cup

-
 Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill
-
Swiss racer Von Allmen wins first gold of Winter Olympics

-
 'Wake up': Mum sparks comeback after scare for freeski star Gu
'Wake up': Mum sparks comeback after scare for freeski star Gu
-
Von Allmen wins men's Olympic downhill gold, first of Games

-
 First medals up for grabs at Winter Olympics
First medals up for grabs at Winter Olympics
-
Afghanistan captain Khan harbours dream of playing in Kabul

-
 Lindsey Vonn completes second Winter Olympics downhill training run
Lindsey Vonn completes second Winter Olympics downhill training run
-
Freeski star Gu survives major scare in Olympic slopestyle

-
 Iran FM looks to more nuclear talks, but warns US
Iran FM looks to more nuclear talks, but warns US
-
Hetmyer's six-hitting steers West Indies to 182-5 against Scotland

-
 After boos for Vance, IOC says it hopes for 'fair play'
After boos for Vance, IOC says it hopes for 'fair play'
-
Thousands gather as Pakistan buries victims of mosque suicide attack

-
 Lindsey Vonn completes second downhill training session
Lindsey Vonn completes second downhill training session
-
US pressing Ukraine and Russia to end war by June, Zelensky says

-
 Faheem blitz sees Pakistan avoid Netherlands shock at T20 World Cup
Faheem blitz sees Pakistan avoid Netherlands shock at T20 World Cup
-
Takaichi talks tough on immigration on eve of vote

-
 England's Salt passed fit for T20 World Cup opener
England's Salt passed fit for T20 World Cup opener
-
Spain, Portugal brace for fresh storm after flood deaths

-
 Pakistan bowl out Netherlands for 147 in T20 World Cup opener
Pakistan bowl out Netherlands for 147 in T20 World Cup opener
-
Pushed to margins, women vanish from Bangladesh's political arena

-
 Crypto firm accidentally sends $40 bn in bitcoin to users
Crypto firm accidentally sends $40 bn in bitcoin to users
-
Pistons end Knicks' NBA winning streak, Celtics edge Heat

-
 Funerals for victims of suicide blast at Islamabad mosque that killed at least 31
Funerals for victims of suicide blast at Islamabad mosque that killed at least 31
-
A tale of two villages: Cambodians lament Thailand's border gains

-
 Police identify suspect in disappearance of Australian boy
Police identify suspect in disappearance of Australian boy
-
Cuba adopts urgent measures to address energy crisis: minister

-
 Not-so-American football: the Super Bowl's overseas stars
Not-so-American football: the Super Bowl's overseas stars
-
Trump says US talks with Iran 'very good,' more negotiations expected

-
 Trump administration re-approves twice-banned pesticide
Trump administration re-approves twice-banned pesticide
-
Hisatsune leads Matsuyama at Phoenix Open as Scheffler makes cut

-
 Beyond the QBs: 5 Super Bowl players to watch
Beyond the QBs: 5 Super Bowl players to watch
-
Grass v artificial turf: Super Bowl players speak out

-
 Police warn Sydney protesters ahead of Israeli president's visit
Police warn Sydney protesters ahead of Israeli president's visit
-
Simi Khanna Launches Simi Beauty SK: A Natural Skincare Line Blending Luxury, Wellness, and Purpose

-
 Best Gold IRA Companies February 2026 Announced (Top Gold-backed IRA Companies Revealed)
Best Gold IRA Companies February 2026 Announced (Top Gold-backed IRA Companies Revealed)
-
Bolivia wants closer US ties, without alienating China: minister


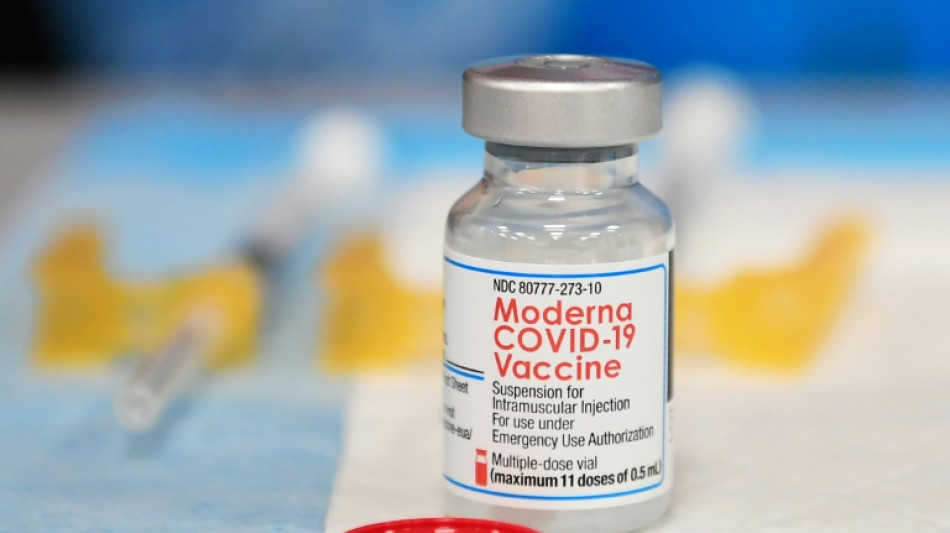
Covid booster efficacy wanes significantly by fourth month: US study
The efficacy of third doses of the Pfizer and Moderna mRNA vaccines wanes substantially by the fourth month after administration, a new study by the US Centers of Disease Control and Prevention (CDC) said Friday.
Though it's now well documented that vaccine efficacy goes down after two doses, relatively little has been published on the duration of protection after a booster.
The new study was based on more than 241,204 visits to the emergency department or an urgent care clinic, and 93,408 hospitalizations, which are more serious, among adults with Covid-19–like illness during August 26, 2021–January 22, 2022.
Vaccine efficacy was estimated by comparing the odds of a positive Covid test between vaccinated and unvaccinated patients and using statistical methods to control for calendar week, geographic area, while adjusting for age, the level of local transmission, and patient characteristics like comorbidities.
During the Omicron-predominant period, vaccine efficacy against Covid-associated emergency department or urgent care visits was 87 percent during the two months after a third dose, but fell to 66 percent by the fourth month.
Vaccine efficacy against hospitalization was 91 percent in the first two months, but fell to 78 percent by the fourth month after a third dose.
"The finding that protection conferred by mRNA vaccines waned in the months after receipt of a third vaccine dose reinforces the importance of further consideration of additional doses to sustain or improve protection," the authors concluded.
Speaking at a White House Covid briefing on Wednesday, President Joe Biden's top medical advisor Anthony Fauci said it was likely that fourth doses would more likely be needed for subsets of people who mount weaker immune responses, such as the elderly and immunocompromised.
- New antibody authorized -
In a separate development Friday, the Food and Drug Administration (FDA) authorized a new lab-grown antibody treatment by pharmaceutical company Lilly called bebtelovimab.
The drug is administered as an intravenous injection over at least 30 seconds and has been green lighted for the treatment of mild-to-moderate Covid among people 12 and over at high risk of severe disease.
Data supporting the authorization came from a clinical trial that showed the drug has strong promise against Omicron. Lilly's previous antibody treatment was de-authorized by the FDA after it was found to be ineffective against this variant.
F.Bennett--AMWN


