-
 Iran vows no surrender as air strikes hit Tehran airport
Iran vows no surrender as air strikes hit Tehran airport
-
Hamilton says 'not where we wanted or expected' for Australian GP

-
 Pole-sitter Russell says his Mercedes more go-kart than 'bouncing bus'
Pole-sitter Russell says his Mercedes more go-kart than 'bouncing bus'
-
Google gives CEO new pay deal worth up to $692 million

-
 Thousands of Taiwan fans turn Tokyo blue at World Baseball Classic
Thousands of Taiwan fans turn Tokyo blue at World Baseball Classic
-
Verstappen baffled by crash in Australian Grand Prix qualifying

-
 Russell leads Mercedes 1-2 for Australian GP as Verstappen crashes
Russell leads Mercedes 1-2 for Australian GP as Verstappen crashes
-
'Grateful' Osaka returns to action with Indian Wells win

-
 Israel fires 'broad-scale' strikes on Tehran as war hits 2nd week
Israel fires 'broad-scale' strikes on Tehran as war hits 2nd week
-
Rapper-turned-politician looks set for landslide Nepal election win

-
 Russian strike on Kharkiv apartment block kills three
Russian strike on Kharkiv apartment block kills three
-
Judge homers as USA cruise past Brazil in World Baseball Classic

-
 Russian strike on Kharkiv appartment block kills three
Russian strike on Kharkiv appartment block kills three
-
Grabbing the bull by the tail: Venezuela's cowboy sport

-
 Russell tops final practice in Melbourne as Antonelli crashes heavily
Russell tops final practice in Melbourne as Antonelli crashes heavily
-
Vibes war? Trump pitches Iran conflict on 'feeling'

-
 Nepal's rapper-turned-politician looks set for landslide win
Nepal's rapper-turned-politician looks set for landslide win
-
Tatum's 'emotional' return sparks Celtics over Mavs

-
 Rising US fuel prices risk sparking domestic wildfire for Trump
Rising US fuel prices risk sparking domestic wildfire for Trump
-
Questions over AI capability as tech guides Iran strikes

-
 Trump convenes Latin American leaders to curb crime, immigration
Trump convenes Latin American leaders to curb crime, immigration
-
Venezuela inflation hit 475% in 2025, the world's highest level

-
 Only Iran's 'unconditional surrender' can end war: Trump
Only Iran's 'unconditional surrender' can end war: Trump
-
Former 100m champion Kerley banned two years over whereabouts failures

-
 Sabalenka opens Indian Wells bid with dominant win
Sabalenka opens Indian Wells bid with dominant win
-
Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh

-
 Man City aren't a 'complete team' admits Guardiola
Man City aren't a 'complete team' admits Guardiola
-
Arteta warns Arsenal to preserve reputation in Mansfield clash

-
 Timothee Chalamet taken to task over opera, ballet dig
Timothee Chalamet taken to task over opera, ballet dig
-
Ireland keep title hopes alive in thrilling win over Wales

-
 Hungary has not returned cash seized from bank workers, Kyiv says
Hungary has not returned cash seized from bank workers, Kyiv says
-
Napoli secure first Serie A home win since January

-
 Valverde strikes late as Real Madrid beat Celta Vigo
Valverde strikes late as Real Madrid beat Celta Vigo
-
PSG beaten by Monaco ahead of Chelsea Champions League showdown

-
 Liverpool tame Wolves to reach FA Cup quarter-finals
Liverpool tame Wolves to reach FA Cup quarter-finals
-
Kane-less Bayern brush aside Gladbach to continue title march

-
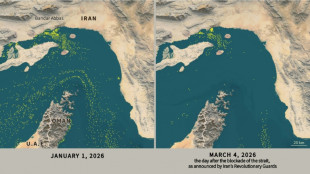 Only nine commercial ships detected crossing Hormuz Strait since Monday
Only nine commercial ships detected crossing Hormuz Strait since Monday
-
Berger extends lead midway through Arnold Palmer Invitational

-
 Paralympics open with Russian athletes booed in ceremony
Paralympics open with Russian athletes booed in ceremony
-
Cuba 'next' on agenda, after Iran: Trump
-
 Zverev leads way into Indian Wells third round
Zverev leads way into Indian Wells third round
-
NASA defense test kicked asteroid off course -- and changed its orbit around the sun

-
 Anthropic vows court fight in Pentagon row
Anthropic vows court fight in Pentagon row
-
'Harder path': Obama attacks Trump at Jesse Jackson memorial

-
 Amber Glenn says will not visit White House to celebrate Olympic gold
Amber Glenn says will not visit White House to celebrate Olympic gold
-
Russian athletes booed as they parade under own flag at Paralympics opening

-
 Trump to attend return of six US troops killed in Iran war
Trump to attend return of six US troops killed in Iran war
-
Tom Brady flag football event moved from Saudi to Los Angeles: reports

-
 UN chief slams 'unlawful attacks', says Mideast could spiral out of control
UN chief slams 'unlawful attacks', says Mideast could spiral out of control
-
Middle East war a new shock for financial markets


IGC Pharma Advances IGC-AD1 as a Potential Alzheimer's Therapy Addressing Cognitive Impairment and Underlying Disease Pathology
New trials to evaluate IGC-AD1's potential impact on amyloid plaque, tau tangles, and cognitive decline in Alzheimer's disease
Expanded research positions IGC-AD1 as a potential treatment targeting the underlying disease pathology of Alzheimer's
New trials to evaluate IGC-AD1's potential impact on amyloid plaque, tau tangles, and cognitive decline in Alzheimer's disease
Expanded research positions IGC-AD1 as a potential treatment targeting the underlying disease pathology of Alzheimer's
IGC Pharma, Inc. (NYSE American:IGC) ("IGC Pharma" or the "Company") announced today an expansion of its clinical research program for IGC-AD1, an investigational treatment for Alzheimer's disease. Building on Phase 2 interim results demonstrating reductions in agitation and cognitive improvement, the Company is initiating new trials to evaluate IGC-AD1's potential as a disease-modifying therapy.
The expanded research will explore how IGC-AD1's dual-action mechanism-combining anti-neuroinflammatory properties with amyloid- and tau-targeting effects-may slow the progression of Alzheimer's disease. These trials will evaluate critical outcomes, including cognitive function and biological markers associated with Alzheimer's, such as amyloid and tau levels, at multiple time points. Building on previously announced preclinical data showing IGC-AD1's impact on amyloid plaques and spatial memory, these investigations aim to explore its potential to influence key pathological features of Alzheimer's disease.
"We plan to initiate a new Phase 2 clinical trial for IGC-AD1 in 2025," said Ram Mukunda, CEO of IGC Pharma. "The trial underscores our commitment to advancing IGC-AD1 as a potential disease-modifying therapy for Alzheimer's. Cognitive decline is one of the most devastating aspects of Alzheimer's, severely impacting patients' memory, attention, and reasoning. By focusing on cognitive outcomes and underlying disease mechanisms such as amyloid plaques and tau tangles, we aim to address the critical unmet needs of patients and caregivers. This subsequent research phase is a significant step forward in delivering innovative treatments to those who need them most.
These new trials represent a pivotal step in advancing IGC-AD1 as a transformative Alzheimer's treatment. By exploring its potential as a disease-modifying therapy, we aim to create opportunities for strategic partnerships and licensing with major pharmaceutical companies. Our vision is to deliver innovative therapies that address the immense challenges faced by patients and caregivers while generating substantial value for our investors."
Building on Promising Preclinical Data
As previously reported, preclinical studies in Alzheimer's mouse models demonstrated an approximate 50% improvement in spatial memory, and cell line data showed a significant 20% reduction in amyloid aggregation following treatment. These preclinical findings, along with the interim Phase 2 data on cognition, provide a solid scientific basis for the expanded research program, which aims to validate IGC-AD1's ability to address key pathological features of Alzheimer's disease.
The ongoing 146-patient Phase 2 trial continues to enroll participants across the USA and Canada. With over 1,000 doses administered and no serious adverse events reported, the trial is on track to deliver comprehensive safety and efficacy data in 2025.
About IGC Pharma (dba IGC):
IGC Pharma is an AI-powered, clinical-stage biotechnology company focused on developing innovative treatments for Alzheimer's disease and transforming patient care with fast-acting, safe, and effective solutions. Our portfolio includes the TGR family, including TGR-63, which targets amyloid plaques, a hallmark of Alzheimer's. The IGC-C and IGC-M platforms are advancing in preclinical studies, focusing on metabolic disorders, tau proteins, early plaque formation, and multiple disease hallmarks. Our lead therapeutic candidate, IGC-AD1, is a cannabinoid-based treatment currently in a Phase 2 trial for agitation in dementia associated with Alzheimer's (clinicaltrials.gov, NCT05543681). Clinical data for IGC-AD1 demonstrated that it has the potential to transform patient care by offering faster-acting and more effective relief compared to traditional medications. Additionally, our AI models are designed to predict potential biomarkers for the early detection of Alzheimer's, optimize clinical trials, and predict receptor affinity, among others. With 28 patent filings and a commitment to innovation, IGC Pharma is dedicated to advancing pharmaceutical treatments and improving the lives of those affected by Alzheimer's and related conditions.
Forward-looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 24, 2024, and on Form 10-Q filed with the SEC on August 7, 2024, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur.
Contact Information
Rosalyn Christian / Walter Frank
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
F.Pedersen--AMWN
