
-
 Venezuela inflation hit 475% in 2025, the world's highest level
Venezuela inflation hit 475% in 2025, the world's highest level
-
Only Iran's 'unconditional surrender' can end war: Trump

-
 Former 100m champion Kerley banned two years over whereabouts failures
Former 100m champion Kerley banned two years over whereabouts failures
-
Sabalenka opens Indian Wells bid with dominant win

-
 Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh
Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh
-
Man City aren't a 'complete team' admits Guardiola

-
 Arteta warns Arsenal to preserve reputation in Mansfield clash
Arteta warns Arsenal to preserve reputation in Mansfield clash
-
Timothee Chalamet taken to task over opera, ballet dig

-
 Ireland keep title hopes alive in thrilling win over Wales
Ireland keep title hopes alive in thrilling win over Wales
-
Hungary has not returned cash seized from bank workers, Kyiv says

-
 Napoli secure first Serie A home win since January
Napoli secure first Serie A home win since January
-
Valverde strikes late as Real Madrid beat Celta Vigo

-
 PSG beaten by Monaco ahead of Chelsea Champions League showdown
PSG beaten by Monaco ahead of Chelsea Champions League showdown
-
Liverpool tame Wolves to reach FA Cup quarter-finals

-
 Kane-less Bayern brush aside Gladbach to continue title march
Kane-less Bayern brush aside Gladbach to continue title march
-
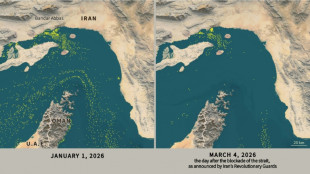
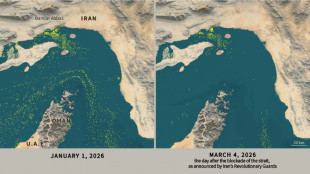
Only nine commercial ships detected crossing Hormuz Strait since Monday
-
 Berger extends lead midway through Arnold Palmer Invitational
Berger extends lead midway through Arnold Palmer Invitational
-
Paralympics open with Russian athletes booed in ceremony

-
 Cuba 'next' on agenda, after Iran: Trump
Cuba 'next' on agenda, after Iran: Trump
-
Zverev leads way into Indian Wells third round

-
 NASA defense test kicked asteroid off course -- and changed its orbit around the sun
NASA defense test kicked asteroid off course -- and changed its orbit around the sun
-
Anthropic vows court fight in Pentagon row

-
 'Harder path': Obama attacks Trump at Jesse Jackson memorial
'Harder path': Obama attacks Trump at Jesse Jackson memorial
-
Amber Glenn says will not visit White House to celebrate Olympic gold

-
 Russian athletes booed as they parade under own flag at Paralympics opening
Russian athletes booed as they parade under own flag at Paralympics opening
-
Trump to attend return of six US troops killed in Iran war

-
 Tom Brady flag football event moved from Saudi to Los Angeles: reports
Tom Brady flag football event moved from Saudi to Los Angeles: reports
-
UN chief slams 'unlawful attacks', says Mideast could spiral out of control

-
 Middle East war a new shock for financial markets
Middle East war a new shock for financial markets
-
Only nine commercial ships detected crossing the Hormuz Strait since Monday
-
 Mexico unveils 100,000-strong security deployment for World Cup
Mexico unveils 100,000-strong security deployment for World Cup
-
Trump's Iran war violates international law, experts say

-
 Swiss eyeing fewer F-35 fighters, reshaping defence set-up
Swiss eyeing fewer F-35 fighters, reshaping defence set-up
-
UK police question three women in Al-Fayed probe

-
 Oil prices surge as Mideast war rages, stocks fall on US jobs
Oil prices surge as Mideast war rages, stocks fall on US jobs
-
Dupont says France must forget Six Nations title talk against Scotland

-
 Voices from Iran: protests, fear and scarcity
Voices from Iran: protests, fear and scarcity
-
Champions League ambitions encourage Barca gamble in Bilbao

-
 This is how Ukraine has countered Russia's Iran-designed drones
This is how Ukraine has countered Russia's Iran-designed drones
-
Dybala out for six weeks as Roma battle for top-four spot

-
 Sleepless Iranians count cost of war as damage mounts
Sleepless Iranians count cost of war as damage mounts
-
Itoje tells faltering England to 'take the game to Italy' in Six Nations

-
 Leading satellite firm to hold back Gulf state images
Leading satellite firm to hold back Gulf state images
-
Tuipulotu urges Scotland to stay in Six Nations title hunt against France

-
 Trump says only Iran's 'unconditional surrender' can end war
Trump says only Iran's 'unconditional surrender' can end war
-
US releases Epstein files with uncorroborated Trump allegations

-
 Securing shipping lane from Mideast war 'challenging', say experts
Securing shipping lane from Mideast war 'challenging', say experts
-
Italy have to start beating the best, says captain Lamaro

-
 US retail sales decline as consumer pullback deepens
US retail sales decline as consumer pullback deepens
-
War in Middle East raises stagflation fears in Europe and beyond


enVVeno Medical Successfully Completes Final Wave of Implants in Pre-Clinical GLP Study for enVVe
Successful Completion of All Planned Implants in GLP Study
enVVe Delivery System Demonstrates Consistent Performance
Company Maintains Timeline for IDE Application Submission by Mid-2025, Pending GLP Study Results
enVVeno Medical Corporation (Nasdaq:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of venous disease, today announced the successful completion of the final wave of implants for shorter-term subjects in its six-month pre-clinical GLP study for enVVe, its transcatheter-delivered replacement venous valve.
The successful completion of all planned implants in the GLP study, including both long-term and short-term subjects, completes a critical phase of the study. The follow-up period, which began with the first wave of implants, is ongoing as scheduled. Pending successful completion of the GLP study, the Company anticipates submitting its IDE application to the FDA in mid-2025. The submission, if approved, would allow the Company to initiate the pivotal clinical trial for enVVe.
"With the successful completion of all planned implants in the enVVe GLP study, we have achieved the last of our milestones for 2024," said Robert Berman, enVVeno Medical's Chief Executive Officer. "Our enhanced enVVe crimping and delivery system has performed very well throughout the study and is ready for the pivotal trial. We will continue to monitor the performance of the enVVe valves throughout the remainder of the study and with successful data and pathology, should be in a position to file the IDE on schedule in mid 2025. We remain focused on our goal of becoming the established leader in both the surgical and non-surgical replacement venous valve markets for patients with severe deep venous CVI."
Severe, deep venous Chronic Venous Insufficiency (CVI) is a debilitating disease that is most often caused by blood clots (deep vein thromboses or DVTs) in the deep veins of the leg. When valves inside of the veins of the leg fail, blood flows in the wrong direction and pools in the lower leg, causing pressure within the veins of the leg to increase (venous hypertension). Symptoms of severe CVI include leg swelling, pain, edema, and in the most severe cases, recurrent open sores known as venous ulcers. The disease can severely impact everyday functions such as sleeping, bathing, dressing, and walking, and is known to result in high rates of depression and anxiety. There are currently no effective treatments for severe CVI of the deep vein system caused by valvular incompetence. Estimates indicate that CVI costs the U.S. healthcare system in excess of $4 billion each year.
The Company's lead product is the VenoValve,® a potential first-in-class, surgical replacement venous valve for patients with severe deep venous CVI. In November, the Company submitted a PMA application with the U.S. Food and Drug Administration seeking approval to market and sell the VenoValve in the U.S. The Company estimates that there are approximately 2.5 million potential new patients each year in the U.S. that could be candidates for the VenoValve. The Company is also developing enVVe®, a next-generation, transcatheter based replacement venous valve, that could appeal to an even larger market in terms of both patients and physicians.
Beginning early next year, the Company will begin to implement its strategy to transition from a development stage to a commercial entity for the VenoValve, while completing the necessary non-clinical and GLP testing for enVVe in preparation for its IDE application.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ:NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. CVI occurs when valves inside of the veins of the leg become damaged, resulting in the backwards flow of blood (reflux), blood pooling in the lower leg, increased pressure in the veins of the leg (venous hypertension) and in severe cases, venous ulcers that are difficult to heal and become chronic. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The VenoValve is currently being evaluated in the SAVVE U.S. pivotal study and the Company is currently performing the final testing necessary to seek approval for the pivotal trial for enVVe.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing (may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
###
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
SOURCE: enVVeno Medical Corporation
P.Stevenson--AMWN

