
-
 EU warns against long war, urges 'credible transition' in Iran
EU warns against long war, urges 'credible transition' in Iran
-
Bored of peace? Trump keeps choosing war

-
 Arteta embraces Arsenal's 'Set-Piece FC' label after corners sink Chelsea
Arteta embraces Arsenal's 'Set-Piece FC' label after corners sink Chelsea
-
Sevilla rescue derby draw to deal Betis top four setback

-
 India need 'special effort' to beat England in semi-final: Gambhir
India need 'special effort' to beat England in semi-final: Gambhir
-
'A terrible day,' says Israel community shaken by deadly Iranian strike

-
 Arsenal corner Chelsea into submission, Man Utd climb to third
Arsenal corner Chelsea into submission, Man Utd climb to third
-
Arsenal win set-piece battle to sink Chelsea in title boost

-
 What future for Iranian leadership after Khamenei's death?
What future for Iranian leadership after Khamenei's death?
-
'Scream 7' makes a killing at N. America box office

-
 Thousands stranded as Iran conflict shuts Mideast hubs
Thousands stranded as Iran conflict shuts Mideast hubs
-
Samson's 97 puts India into T20 World Cup semi-final against England

-
 Latest developments as Iran retaliates to US-Israel strikes that killed Khamenei
Latest developments as Iran retaliates to US-Israel strikes that killed Khamenei
-
Spurs have 'big problems' says Tudor as relegation risk persists

-
 Dortmund captain Can out for season with ACL tear
Dortmund captain Can out for season with ACL tear
-
Leweling doubles up as Stuttgart sink sorry Wolfsburg

-
 Man Utd climb to third, Fulham sink sorry Spurs
Man Utd climb to third, Fulham sink sorry Spurs
-
Iran strikes send VIP Dubai influencers 'back to reality'

-
 Briton Brennan bursts to Kuurne-Bruxelles-Kuurne triumph
Briton Brennan bursts to Kuurne-Bruxelles-Kuurne triumph
-
Activists pressure Milan Fashion Week to go fully fur-free

-
 Blasts in Kabul as Afghan govt says responding to Pakistan attacks
Blasts in Kabul as Afghan govt says responding to Pakistan attacks
-
Iranians grieve, celebrate, worry after Khamenei's killing

-
 Latest developments as Iran lashes out after US-Israel strikes kill Khamenei
Latest developments as Iran lashes out after US-Israel strikes kill Khamenei
-
West Indies post 195-4 against India in T20 World Cup do-or-die clash

-
 South Africa 'embrace pressure' and favourites tag, says coach
South Africa 'embrace pressure' and favourites tag, says coach
-
Tel Aviv residents say ready to withstand more Iranian attacks

-
 Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
-
AC Milan consolidate top-four credentials with win at Cremonese

-
 Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
-
Guardiola calls for respect after Ramadan break is booed

-
 Afghanistan warns Iran war will impact whole region
Afghanistan warns Iran war will impact whole region
-
Iran launches fresh strikes across Gulf after vowing revenge for slain leader

-
 OPEC+ hikes oil production by more than expected following outbreak of Iran war
OPEC+ hikes oil production by more than expected following outbreak of Iran war
-
Goggia tightens grip on World Cup super-G with victory in Andorra

-
 Belgium seizes Russian 'shadow fleet' tanker
Belgium seizes Russian 'shadow fleet' tanker
-
Raza steers Zimbabwe to 153-7 against South Africa

-
 Kerr on target as Australia make winning start to Women's Asian Cup
Kerr on target as Australia make winning start to Women's Asian Cup
-
Marquez says 'unlucky' to retire from MotoGP season opener

-
 9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
-
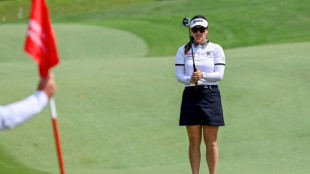
Green clinches Singapore title with help from caddie husband
-
 More flights cancelled as Iran conflict shuts Mideast hubs
More flights cancelled as Iran conflict shuts Mideast hubs
-
'One Battle After Another' wins top producer award before Oscars

-
 Iran vows revenge for slain supreme leader despite Trump threat
Iran vows revenge for slain supreme leader despite Trump threat
-
Flights of fancy at Bottega Veneta with shimmering, tactile collection

-
 World Cup marks 100-day countdown amid political upheaval
World Cup marks 100-day countdown amid political upheaval
-
Bezzecchi wins MotoGP opener as Marquez retires

-
 Pro-Iran protesters try to storm US missions in Pakistan, Iraq
Pro-Iran protesters try to storm US missions in Pakistan, Iraq
-
8 killed in pro-Iran protest at US consulate in Pakistan's Karachi

-
 Latest developments after US, Israeli strikes kill Iran's Khamenei
Latest developments after US, Israeli strikes kill Iran's Khamenei
-
Before dawn, ancient drum rite wakes Istanbul faithful to fast


VenoValve to be Featured During Presentation at the 47th Annual Charing Cross Symposium
IRVINE, CA / ACCESS Newswire / April 23, 2025 / enVVeno Medical Corporation (Nasdaq:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of deep venous disease, today announced that the VenoValve® will be featured during a presentation at the Annual Charing Cross Symposium being held April 23-25, 2025 in London, England.
As part of the Symposium, Dr. David Dexter, Sentara Hospital, Norfolk, Virginia and Associate Professor of Surgery, Eastern Virginia Medical School, and a Principal Investigator for the VenoValve U.S. pivotal trial, will present, "Progress to date and future prospects for invasive correction of deep venous reflux" onThursday, April 24, 2025 at 11:10 AM BST.
The VenoValveis a potential first-in-class, surgical replacement venous valve for patients with severe deep venous CVI. The Company estimates that there are approximately 2.5 million potential new patients each year in the U.S. that could be candidates for the VenoValve. The Company has submitted a pre-market authorization (PMA) application for the VenoValve to the U.S. Food and Drug Administration (FDA), with a decision anticipated in the second half of 2025.
For more information, visit the Annual Charing Cross Symposium website here.
About CVI
Severe, deep venous Chronic Venous Insufficiency (CVI) is a debilitating disease that is most often caused by blood clots (deep vein thromboses or DVTs) in the deep veins of the leg. When valves inside of the veins of the leg fail, blood flows in the wrong direction and pools in the lower leg, causing pressure within the veins of the leg to increase (venous hypertension). Symptoms of severe CVI include leg swelling, pain, edema, and in the most severe cases, recurrent open sores known as venous ulcers. The disease can severely impact everyday functions such as sleeping, bathing, dressing, and walking, and is known to result in high rates of depression and anxiety. There are currently no effective treatments for severe CVI of the deep vein system caused by valvular incompetence. Estimates indicate that CVI costs the U.S. healthcare system in excess of $4 billion each year.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ:NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of deep venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. CVI occurs when valves inside of the veins of the leg become damaged, resulting in the backwards flow of blood (reflux), blood pooling in the lower leg, increased pressure in the veins of the leg (venous hypertension) and in severe cases, venous ulcers that are difficult to heal and become chronic. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The VenoValve is currently being evaluated in the SAVVE U.S. pivotal study and the Company is currently performing the final testing necessary to seek approval for the pivotal trial for enVVe.
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
MEDIA CONTACT:
Glenn Silver, FINN Partners
[email protected]
(973) 818-8198
SOURCE: enVVeno Medical Corporation
View the original press release on ACCESS Newswire
F.Schneider--AMWN



