
-
 Dortmund captain Can out for season with ACL tear
Dortmund captain Can out for season with ACL tear
-
Leweling doubles up as Stuttgart sink sorry Wolfsburg

-
 Man Utd climb to third, Fulham sink sorry Spurs
Man Utd climb to third, Fulham sink sorry Spurs
-
Iran strikes send VIP Dubai influencers 'back to reality'

-
 Briton Brennan bursts to Kuurne-Bruxelles-Kuurne triumph
Briton Brennan bursts to Kuurne-Bruxelles-Kuurne triumph
-
Activists pressure Milan Fashion Week to go fully fur-free

-
 Blasts in Kabul as Afghan govt says responding to Pakistan attacks
Blasts in Kabul as Afghan govt says responding to Pakistan attacks
-
Iranians grieve, celebrate, worry after Khamenei's killing

-
 Latest developments as Iran lashes out after US-Israel strikes kill Khamenei
Latest developments as Iran lashes out after US-Israel strikes kill Khamenei
-
West Indies post 195-4 against India in T20 World Cup do-or-die clash

-
 South Africa 'embrace pressure' and favourites tag, says coach
South Africa 'embrace pressure' and favourites tag, says coach
-
Tel Aviv residents say ready to withstand more Iranian attacks

-
 Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
-
AC Milan consolidate top-four credentials with win at Cremonese

-
 Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
-
Guardiola calls for respect after Ramadan break is booed

-
 Afghanistan warns Iran war will impact whole region
Afghanistan warns Iran war will impact whole region
-
Iran launches fresh strikes across Gulf after vowing revenge for slain leader

-
 OPEC+ hikes oil production by more than expected following outbreak of Iran war
OPEC+ hikes oil production by more than expected following outbreak of Iran war
-
Goggia tightens grip on World Cup super-G with victory in Andorra

-
 Belgium seizes Russian 'shadow fleet' tanker
Belgium seizes Russian 'shadow fleet' tanker
-
Raza steers Zimbabwe to 153-7 against South Africa

-
 Kerr on target as Australia make winning start to Women's Asian Cup
Kerr on target as Australia make winning start to Women's Asian Cup
-
Marquez says 'unlucky' to retire from MotoGP season opener

-
 9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
-
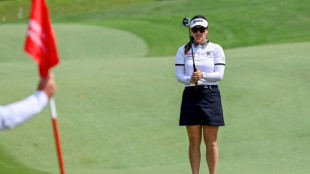
Green clinches Singapore title with help from caddie husband
-
 More flights cancelled as Iran conflict shuts Mideast hubs
More flights cancelled as Iran conflict shuts Mideast hubs
-
'One Battle After Another' wins top producer award before Oscars

-
 Iran vows revenge for slain supreme leader despite Trump threat
Iran vows revenge for slain supreme leader despite Trump threat
-
Flights of fancy at Bottega Veneta with shimmering, tactile collection

-
 World Cup marks 100-day countdown amid political upheaval
World Cup marks 100-day countdown amid political upheaval
-
Bezzecchi wins MotoGP opener as Marquez retires

-
 Pro-Iran protesters try to storm US missions in Pakistan, Iraq
Pro-Iran protesters try to storm US missions in Pakistan, Iraq
-
8 killed in pro-Iran protest at US consulate in Pakistan's Karachi

-
 Latest developments after US, Israeli strikes kill Iran's Khamenei
Latest developments after US, Israeli strikes kill Iran's Khamenei
-
Before dawn, ancient drum rite wakes Istanbul faithful to fast

-
 Music, mourning as Iran's Khamenei is killed
Music, mourning as Iran's Khamenei is killed
-
Pakistan cricket's lack of T20 evolution exposed by World Cup exit

-
 Cobolli downs Tiafoe to claim Mexican Open
Cobolli downs Tiafoe to claim Mexican Open
-
Takele defends Tokyo Marathon title after sprint finish

-
 Hollywood's finest gather for guild's Actor Awards
Hollywood's finest gather for guild's Actor Awards
-
Iran prepare for Women's Asian Cup as bombs drop on homeland

-
 Doncic shines as Lakers cruise past depleted Warriors
Doncic shines as Lakers cruise past depleted Warriors
-
3D tool Unreal Engine makes real impact in creative industries

-
 OPEC+ mulls oil production increase in shadow of war
OPEC+ mulls oil production increase in shadow of war
-
Putin, Russia's eternal leader defined by war and power

-
 Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
-
Iranians across North America rally for -- and against -- strikes

-
 Shakespeare would have shunned streaming, 'Hamnet' team says
Shakespeare would have shunned streaming, 'Hamnet' team says
-
Will Oscars be 17th time lucky for songwriter Diane Warren?


Avant Technologies and Ainnova Request Pre-Submission Meeting with US FDA for VisionAI Platform Technology
LAS VEGAS, NEVADA / ACCESS Newswire / April 29, 2025 / Avant Technologies Inc. (OTCQB:AVAI) ("Avant" or the "Company"), and its JV partner, Ainnova Tech, Inc., (Ainnova), a leading healthcare technology company focused on revolutionizing early disease detection using artificial intelligence (AI), today announced that The Center for Devices and Radiological Health of the U.S. Food and Drug Administration (FDA) has received the company's submission package requesting a pre-submission meeting with the FDA for its VisionAI platform technology and is now under review.
Ainnova is requesting a pre-submission meeting with the FDA's review team to discuss any questions and/or concerns about its proposed formal submission, including seeking advice to finalize the protocol and obtain agency guidance for a clinical trial of its VisionAI platform in the early detection of diabetic retinopathy. A pre-submission meeting allows companies to clarify regulatory requirements, get feedback on their plans, and potentially avoid delays or issues during the formal review process.
The clinical studies will aim to support an FDA 510(k) submission to obtain clearance from the regulatory agency to market its technology in the U.S.
Ai-nova Acquisition Corp. (AAC), the company formed by the partnership between Avant and Ainnova to advance and commercialize Ainnova's technology portfolio, including its VisionAI platform and its versatile retinal cameras, has worldwide licensing rights for this portfolio. The licensing rights include the U.S., where the FDA regulates drug and medical device development, so the success of Ainnova's interactions with the FDA are paramount to marketing the technology portfolio in the United States.
Vinicio Vargas, Chief Executive Officer at Ainnova and a member of AAC's Board of Directors, said, "This milestone reflects our two-tiered strategy, rapid deployment in low-regulation markets where VisionAI operates as a screening tool, and simultaneous progress toward FDA clearance for the U.S. market. Entering the U.S. will unlock significant commercial potential, and early engagement with regulators ensures we do so with speed, credibility, and a validated product."
For medical device applicants like Ainnova, the FDA's pre-submission program is useful to determine a clear regulatory pathway for the successful launch of the device, including the number of patients and the number of clinics that will be needed to generate the necessary clinical data for the FDA to make an informed decision on Ainnova's VisionAI platform. For Avant, the pre-submission meeting will help define a precise budget for the strategic partnership's entire FDA process.
About Ainnova Tech, Inc.
Ainnova is a Nevada-based healthtech startup with headquarters in San Jose, Costa Rica, and Houston, Texas. Founded by an experienced and innovative team that is dedicated to leveraging artificial intelligence for early disease detection. Recognized with multiple global awards and renowned partnerships with hospitals and medical device companies, we proudly introduce VisionAI - our cutting-edge platform designed to prevent blindness and detect the early onset of diabetes. Explore how Ainnova is revolutionizing healthcare through advanced technology and proactive solutions.
About Avant Technologies Inc.
Avant Technologies, Inc. is an emerging technology company developing solutions in artificial intelligence in healthcare. With a focus on pushing the boundaries of what is possible in AI and machine learning, Avant serves a diverse range of industries, driving progress and efficiency through state-of-the-art technology.
More information about Avant can be found at https://avanttechnologies.com
You can also follow us on social media at:
https://twitter.com/AvantTechAI
https://www.linkedin.com/company/avant-technologies-ai
https://www.facebook.com/AvantTechAI
https://www.youtube.com/@AvantTechAI
Forward-Looking Statements
Certain statements contained in this press release may constitute "forward-looking statements." Forward-looking statements provide current expectations of future events based on certain assumptions and include any statement that does not directly relate to any historical or current fact. Actual results may differ materially from those indicated by such forward-looking statements because of various important factors as disclosed in our filings with the Securities and Exchange Commission located at their website (http://www.sec.gov). In addition to these factors, actual future performance, outcomes, and results may differ materially because of more general factors including (without limitation) general industry and market conditions and growth rates, economic conditions, governmental and public policy changes, the Company's ability to raise capital on acceptable terms, if at all, the Company's successful development of its products and the integration into its existing products and the commercial acceptance of the Company's products. The forward-looking statements included in this press release represent the Company's views as of the date of this press release and these views could change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company's views as of any date after the date of the press release.
Contact:
Avant Technologies Inc.
[email protected]
SOURCE: Avant Technologies
View the original press release on ACCESS Newswire
A.Mahlangu--AMWN


