
-
 South Africa 'embrace pressure' and favourites tag, says coach
South Africa 'embrace pressure' and favourites tag, says coach
-
Tel Aviv residents say ready to withstand more Iranian attacks

-
 Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
Russia loses key ally leader as Putin slams Khamenei 'cynical' killing
-
AC Milan consolidate top-four credentials with win at Cremonese

-
 Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
-
Guardiola calls for respect after Ramadan break is booed

-
 Afghanistan warns Iran war will impact whole region
Afghanistan warns Iran war will impact whole region
-
Iran launches fresh strikes across Gulf after vowing revenge for slain leader

-
 OPEC+ hikes oil production by more than expected following outbreak of Iran war
OPEC+ hikes oil production by more than expected following outbreak of Iran war
-
Goggia tightens grip on World Cup super-G with victory in Andorra

-
 Belgium seizes Russian 'shadow fleet' tanker
Belgium seizes Russian 'shadow fleet' tanker
-
Raza steers Zimbabwe to 153-7 against South Africa

-
 Kerr on target as Australia make winning start to Women's Asian Cup
Kerr on target as Australia make winning start to Women's Asian Cup
-
Marquez says 'unlucky' to retire from MotoGP season opener

-
 9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
-
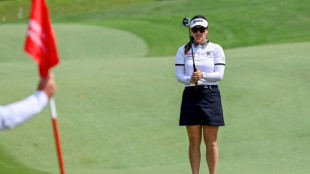
Green clinches Singapore title with help from caddie husband
-
 More flights cancelled as Iran conflict shuts Mideast hubs
More flights cancelled as Iran conflict shuts Mideast hubs
-
'One Battle After Another' wins top producer award before Oscars

-
 Iran vows revenge for slain supreme leader despite Trump threat
Iran vows revenge for slain supreme leader despite Trump threat
-
Flights of fancy at Bottega Veneta with shimmering, tactile collection

-
 World Cup marks 100-day countdown amid political upheaval
World Cup marks 100-day countdown amid political upheaval
-
Bezzecchi wins MotoGP opener as Marquez retires

-
 Pro-Iran protesters try to storm US missions in Pakistan, Iraq
Pro-Iran protesters try to storm US missions in Pakistan, Iraq
-
8 killed in pro-Iran protest at US consulate in Pakistan's Karachi

-
 Latest developments after US, Israeli strikes kill Iran's Khamenei
Latest developments after US, Israeli strikes kill Iran's Khamenei
-
Before dawn, ancient drum rite wakes Istanbul faithful to fast

-
 Music, mourning as Iran's Khamenei is killed
Music, mourning as Iran's Khamenei is killed
-
Pakistan cricket's lack of T20 evolution exposed by World Cup exit

-
 Cobolli downs Tiafoe to claim Mexican Open
Cobolli downs Tiafoe to claim Mexican Open
-
Takele defends Tokyo Marathon title after sprint finish

-
 Hollywood's finest gather for guild's Actor Awards
Hollywood's finest gather for guild's Actor Awards
-
Iran prepare for Women's Asian Cup as bombs drop on homeland

-
 Doncic shines as Lakers cruise past depleted Warriors
Doncic shines as Lakers cruise past depleted Warriors
-
3D tool Unreal Engine makes real impact in creative industries

-
 OPEC+ mulls oil production increase in shadow of war
OPEC+ mulls oil production increase in shadow of war
-
Putin, Russia's eternal leader defined by war and power

-
 Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
-
Iranians across North America rally for -- and against -- strikes

-
 Shakespeare would have shunned streaming, 'Hamnet' team says
Shakespeare would have shunned streaming, 'Hamnet' team says
-
Will Oscars be 17th time lucky for songwriter Diane Warren?

-
 Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
-
Texas port humming as Trump ramps up Venezuela oil

-
 76ers' center Embiid to miss at least three games with oblique strain
76ers' center Embiid to miss at least three games with oblique strain
-
US, Israel defend strikes at UN as Iran alleges 'war crime'

-
 Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
-
Iran attacks rock Dubai's Palm, Burj Al Arab, airport

-
 Sterling Metals Discovers Cu-Mo Porphyry Stock 1.5km from MEPS Discovery Zone Confirming Scale of Porphyry Copper System
Sterling Metals Discovers Cu-Mo Porphyry Stock 1.5km from MEPS Discovery Zone Confirming Scale of Porphyry Copper System
-
JP Anderson Signs Landmark MOU with Vaama Village to Advance Rare Earth Mineral Development in Bonthe District

-
 Iran leader Khamenei killed in massive US and Israeli attack, Trump says
Iran leader Khamenei killed in massive US and Israeli attack, Trump says
-
UK pop-soul star Olivia Dean sweeps Brit Awards


Aspira Reaches Another ARPA-H Milestone, Eligible to Receive Additional $1.5 Million in Second Quarter
Aspira Women's Health Submits the Third Milestone of ARPA-H $10 Million Award
AUSTIN, TX / ACCESS Newswire / May 1, 2025 / Aspira Women's Health Inc. ("Aspira") (OTC PINK:AWHL), a bio-analytical based women's health company focused on the development of gynecologic disease diagnostic tools, today announced the successful submission of its third development milestone of its Advanced Research Projects Agency for Health (ARPA-H) award.
The third milestone focused heavily on additional analytical development steps. The milestone specifically required the completion of additional deliverables including a biomarker technical report, as well as the framework for a voice of customer study. Aspira is now eligible to receive an additional $1.5 million cash payment under the terms of the related $10 million agreement. Payment is expected to be received in the second half of the current fiscal quarter.
"We are very proud to achieve this critical milestone under the ARPA-H award program," commented Mike Buhle, CEO of Aspira. Our team has maintained excellent pace and focus as we work to execute on our development of a groundbreaking new diagnostic for endometriosis through our ENDOinformTM program. Successfully completing this third milestone per the terms of the ARPA-H contract, and ultimately receiving this next payment is a clear and tangible demonstration of the process our technical team has made on advancing ENDOinform."
"Overall, we are scheduled to receive $10 million under the ARPA-H award. We have successfully submitted three milestones worth a total of $3.5 million to date. We are on track to receive our fourth and fifth milestone payments, for a total of $2.0 million at the end of 2025, and the remaining $3.0 million in ARPA-H funding in 2026. This $10 million award has been a key component of our financing strategy, as we continue to work to unlock significant shareholder value through our ENDOinform development program," concluded Mr. Buhle.
ARPA-H's Sprint for Women's Health was created to address critical unmet challenges in women's health, champion transformative innovations, and tackle health conditions that uniquely or disproportionately affect women. Aspira is expected to receive a total of $10 million in funding over two years through the Sprint for Women's Health launchpad track for later-stage health solutions. The Company's multi-marker blood test to aid in the detection of endometriosis, which it intends to launch commercially prior to the end of the contract term, will rely on a powerful AI-enabled algorithm that combines protein and microRNA biomarkers and patient data, and leverages technology that Aspira pioneered for its commercially successful ovarian cancer risk assessment blood tests.
About Aspira Women's Health Inc.
Aspira Women's Health Inc. is dedicated to the discovery, development, and commercialization of noninvasive, AI-enabled tests to aid in the diagnosis of gynecologic diseases. OvaWatch® and Ova1Plus® are offered to clinicians as OvaSuiteSM. Together, they provide the only comprehensive portfolio of blood tests to aid in the detection of ovarian cancer risk for the 1.2+ million American women diagnosed with an adnexal mass each year.
OvaWatch provides a negative predictive value of 99% and is used to assess ovarian cancer risk for women where initial clinical assessment indicates the mass is indeterminate or benign, and thus surgery may be premature or unnecessary. Ova1Plus is a reflex process of two FDA-cleared tests, Ova1® and Overa®, to assess the risk of ovarian malignancy in women with an adnexal mass planned for surgery.
Our in-development test pipeline will expand our ovarian cancer portfolio and address the tremendous need for non-invasive diagnostics for endometriosis, a debilitating disease that impacts millions of women worldwide. In ovarian cancer, we intend to combine microRNA and protein biomarkers with patient data to further enhance the sensitivity and specificity of our current tests. In endometriosis, we have developed the first-ever non-invasive test designed to identify endometriomas, one of the most commonly occurring forms of severe endometriosis. Through our ongoing endometriosis development program, we are combining microRNA and protein biomarkers with patient data, with the intent of identifying all endometriosis independent of disease location or severity.
Forward-Looking Statements
This press release contains forward-looking statements, as defined in the Private Securities Litigation Reform Act of 1995. Forward-looking statements involve a number of risks and uncertainties. Such forward-looking statements include statements regarding, among other things, the timing and completion of any products in the development pipeline and other statements that are predictive in nature, and whether the marketing of the OvaSuite portfolio will prove successful. Actual results could differ materially from those discussed due to known and unknown risks, uncertainties, and other factors. These forward-looking statements generally can be identified by the use of words such as "designed to," "expect," "plan," "anticipate," "could," "may," "intend," "will," "continue," "future," and other words of similar meaning and the use of future dates. These and additional risks and uncertainties are described more fully in the Company's filings with the Securities and Exchange Commission (SEC), including those factors identified as "Risk Factors" in our most recent Annual Report on Form 10-K for the fiscal year ended December 31, 2024, and subsequent Quarterly Reports on Form 10-Q. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that Aspira presently does not know, or that Aspira currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect Aspira's expectations, plans, or forecasts of future events and views as of the date of this press release. Subsequent events and developments may cause the Company's assessments to change. However, while Aspira may elect to update these forward-looking statements at some point in the future, Aspira expressly disclaims any obligation to do so, except as required by law. These forward-looking statements should not be relied upon as representing Aspira's assessments of any date after the date of this press release. Accordingly, undue reliance should not be placed upon the forward-looking statements.
Investor Relations Contact:
[email protected]
SOURCE: Aspira Women's Health
View the original press release on ACCESS Newswire
A.Rodriguezv--AMWN


