-
 Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
Flights of fancy at Bottega Veneta, atmospheric mood at Armani in Milan
-
Guardiola calls for respect after Ramadan break is booed

-
 Afghanistan warns Iran war will impact whole region
Afghanistan warns Iran war will impact whole region
-
Iran launches fresh strikes across Gulf after vowing revenge for slain leader

-
 OPEC+ hikes oil production by more than expected following outbreak of Iran war
OPEC+ hikes oil production by more than expected following outbreak of Iran war
-
Goggia tightens grip on World Cup super-G with victory in Andorra

-
 Belgium seizes Russian 'shadow fleet' tanker
Belgium seizes Russian 'shadow fleet' tanker
-
Raza steers Zimbabwe to 153-7 against South Africa

-
 Kerr on target as Australia make winning start to Women's Asian Cup
Kerr on target as Australia make winning start to Women's Asian Cup
-
Marquez says 'unlucky' to retire from MotoGP season opener

-
 9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
9 killed in pro-Iran protest at US consulate in Pakistan's Karachi
-
Green clinches Singapore title with help from caddie husband
-
 More flights cancelled as Iran conflict shuts Mideast hubs
More flights cancelled as Iran conflict shuts Mideast hubs
-
'One Battle After Another' wins top producer award before Oscars

-
 Iran vows revenge for slain supreme leader despite Trump threat
Iran vows revenge for slain supreme leader despite Trump threat
-
Flights of fancy at Bottega Veneta with shimmering, tactile collection

-
 World Cup marks 100-day countdown amid political upheaval
World Cup marks 100-day countdown amid political upheaval
-
Bezzecchi wins MotoGP opener as Marquez retires

-
 Pro-Iran protesters try to storm US missions in Pakistan, Iraq
Pro-Iran protesters try to storm US missions in Pakistan, Iraq
-
8 killed in pro-Iran protest at US consulate in Pakistan's Karachi

-
 Latest developments after US, Israeli strikes kill Iran's Khamenei
Latest developments after US, Israeli strikes kill Iran's Khamenei
-
Before dawn, ancient drum rite wakes Istanbul faithful to fast

-
 Music, mourning as Iran's Khamenei is killed
Music, mourning as Iran's Khamenei is killed
-
Pakistan cricket's lack of T20 evolution exposed by World Cup exit

-
 Cobolli downs Tiafoe to claim Mexican Open
Cobolli downs Tiafoe to claim Mexican Open
-
Takele defends Tokyo Marathon title after sprint finish

-
 Hollywood's finest gather for guild's Actor Awards
Hollywood's finest gather for guild's Actor Awards
-
Iran prepare for Women's Asian Cup as bombs drop on homeland

-
 Doncic shines as Lakers cruise past depleted Warriors
Doncic shines as Lakers cruise past depleted Warriors
-
3D tool Unreal Engine makes real impact in creative industries

-
 OPEC+ mulls oil production increase in shadow of war
OPEC+ mulls oil production increase in shadow of war
-
Putin, Russia's eternal leader defined by war and power

-
 Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
Explosion, gunfire as Afghan forces shoot at aircraft over Kabul
-
Iranians across North America rally for -- and against -- strikes

-
 Shakespeare would have shunned streaming, 'Hamnet' team says
Shakespeare would have shunned streaming, 'Hamnet' team says
-
Will Oscars be 17th time lucky for songwriter Diane Warren?

-
 Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
Sympathy for the bedeviled: the likable conspiracy theorist of 'Bugonia'
-
Texas port humming as Trump ramps up Venezuela oil

-
 76ers' center Embiid to miss at least three games with oblique strain
76ers' center Embiid to miss at least three games with oblique strain
-
US, Israel defend strikes at UN as Iran alleges 'war crime'

-
 Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
Brumbies' 'mental resolve' keeps them unbeaten in Super Rugby
-
Iran attacks rock Dubai's Palm, Burj Al Arab, airport

-
 JP Anderson Signs Landmark MOU with Vaama Village to Advance Rare Earth Mineral Development in Bonthe District
JP Anderson Signs Landmark MOU with Vaama Village to Advance Rare Earth Mineral Development in Bonthe District
-
Iran leader Khamenei killed in massive US and Israeli attack, Trump says

-
 UK pop-soul star Olivia Dean sweeps Brit Awards
UK pop-soul star Olivia Dean sweeps Brit Awards
-
Iranians across North America take to the streets for - and against - strikes

-
 'Turning point' as Crusaders notch first Super Rugby win
'Turning point' as Crusaders notch first Super Rugby win
-
White House releases photos of Trump, Vance during Iran ops

-
 PSG win to extend lead over Lens at top of Ligue 1
PSG win to extend lead over Lens at top of Ligue 1
-
Barca's Yamal nets hat-trick in Villarreal romp, Atletico go third


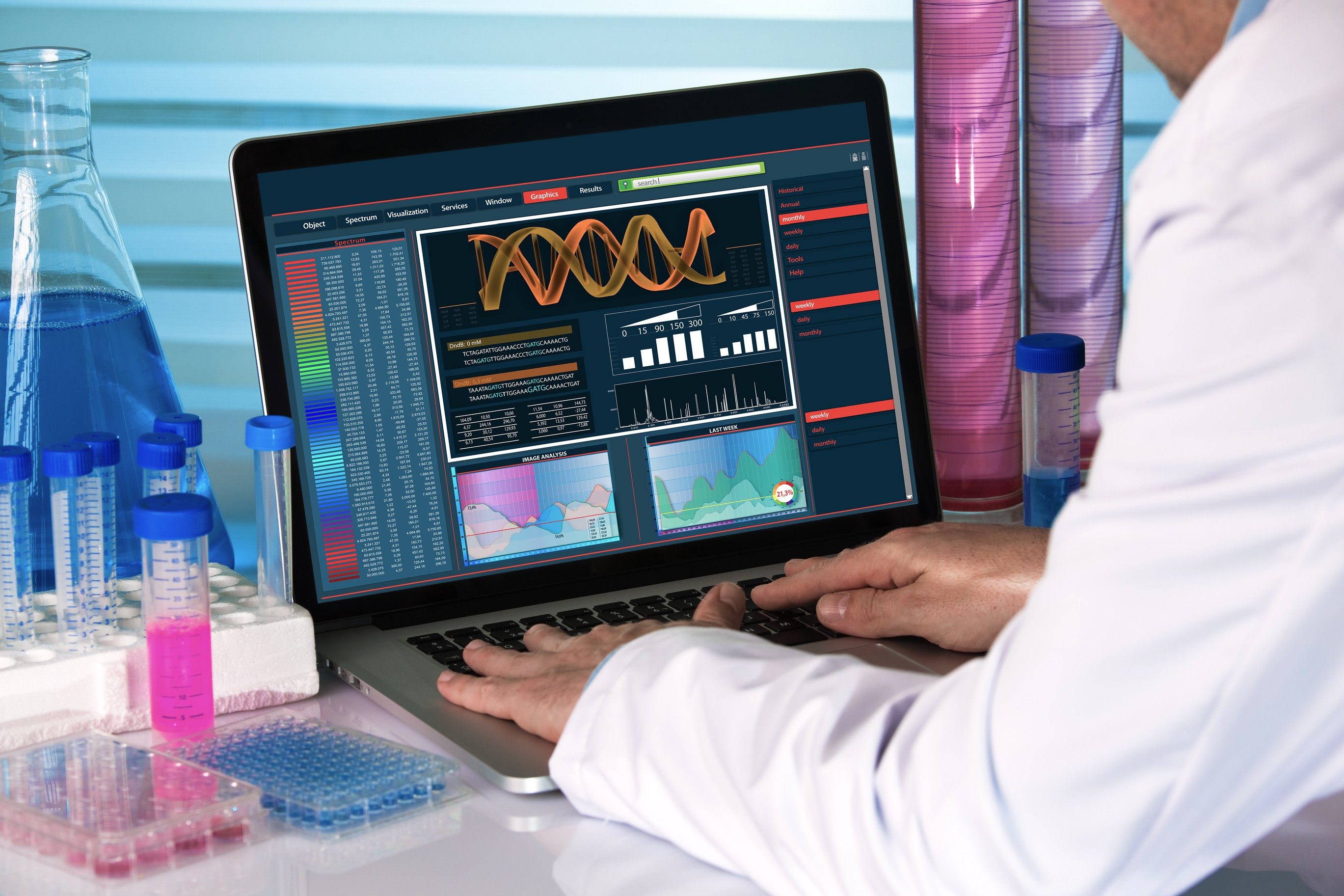
Northway Biotech Launches Full-Service Viral Clearance Studies, Delivering Results Faster Than Industry Standards
With six newly established, identical BSL-2 laboratories now operational, biologics CDMO Northway Biotech can conduct VCS programs for up to six clients simultaneously, significantly alleviating current market bottlenecks
VILNIUS, LT / ACCESS Newswire / May 5, 2025 / Northway Biotech, a biopharmaceutical contract development and manufacturing organization (CDMO), today announced the expansion of its protein-based and gene therapy service offerings with the addition of Viral Clearance Studies (VCS) capabilities. This strategic growth follows the opening of Northway Biotech's new Gene Therapy Center with dedicated cGMP facilities for virus-related projects.
With six newly established, identical BSL-2 laboratories now operational, Northway Biotech can conduct VCS programs for up to six clients simultaneously, significantly alleviating current market bottlenecks. Additionally, the company has expanded its capabilities to perform GMP-compliant manufacturing and testing under BSL-3 conditions, further strengthening its service offering across gene therapy and broader biologics development.
Viral Clearance Studies are now offered both as part of Northway Biotech's integrated CDMO programs and as a standalone service. This flexibility allows external clients to access VCS expertise independently, without requiring a manufacturing agreement.
Accelerated Delivery Timelines - Over One Month Faster Than Industry
Leveraging expanded infrastructure and integrated analytical capabilities, Northway Biotech is positioned to deliver Viral Clearance Studies substantially faster than the current industry standard. Comprehensive studies, assessing viral removal and inactivation, can now be completed with final regulatory-compliant reporting in under 10 weeks from initiation of project design when two model viruses are employed, and within 12 weeks when four model viruses are used.
"Our expansion into Viral Clearance Studies is a natural extension of our CDMO services, enabling us to manage these critical studies in-house and significantly reduce project timelines for our clients," said Prof. Vladas Algirdas Bumelis, CEO of Northway Biotech. "By investing in state-of-the-art BSL-2 and BSL-3 facilities, expanding technical capabilities, and further strengthening our scientific teams, we are uniquely positioned to deliver high-quality VCS data faster - a key advantage for clients advancing through clinical development and regulatory approval."
For more information on Northway Biotech's Viral Clearance Study processes, service offerings, and delivery timelines, please complete the contact form to connect with the Northway Biotech team.
About Northway Biotech - https://www.northwaybiotech.com
Northway Biotech is a leading contract development and manufacturing organization (CDMO) supporting customers worldwide. Its highly experienced and professional team executes projects at every stage, from cell line construction and process development to cGMP manufacturing of biopharmaceutical products. The company's extensive expertise and vertically integrated service offering enables rapid execution of multiple projects from its state-of-the-art GMP facilities while ensuring full process and product compliance at all stages of research, development, and commercial manufacturing. Northway Biotech is a privately owned company founded in 2004 and operates locations in Vilnius, Lithuania; London, United Kingdom; and Waltham, MA, USA.
Media & Business Contact:
Prof. Vladas Algirdas Bumelis
CEO and Chairman of the Board
Northway Biotech
[email protected]
Contact Information
Vladas Bumelis
CEO and Chairman of the Board
[email protected]
SOURCE: Northway Biotech
View the original press release on ACCESS Newswire
J.Williams--AMWN


