
-
 Germany's Aicher wins women's super-G in Soldeu
Germany's Aicher wins women's super-G in Soldeu
-
Fight against terror: Trump threatens Tehran's mullahs

-
 US and Israel launch strikes on Iran, explosions reported across region
US and Israel launch strikes on Iran, explosions reported across region
-
Iran's Khamenei: ruthless revolutionary at apex of Islamic republic

-
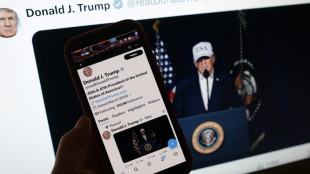 In Iran attack, Trump seeks what he foreswore -- regime change
In Iran attack, Trump seeks what he foreswore -- regime change
-
Climate change forces facelift for Michelangelo masterpiece

-
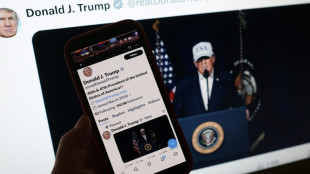 Trump says US aims to destroy Iran's military, topple government
Trump says US aims to destroy Iran's military, topple government
-
Acosta wins season-opening MotoGP sprint after Marquez penalty

-
 US and Israel launch strikes against Iran
US and Israel launch strikes against Iran
-
Afghanistan says Pakistan fighter jet down as cross-border strikes flare

-
 Kerr says only '85 percent' fit for Women's Asian Cup
Kerr says only '85 percent' fit for Women's Asian Cup
-
Messi's Inter Miami to visit White House: US media

-
 Thunder beat Nuggets in overtime on Gilgeous-Alexander's return
Thunder beat Nuggets in overtime on Gilgeous-Alexander's return
-
'It's surreal': Zimbabwe superfans revel in unexpected ride to India

-
 New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania
New 'Wuthering Heights' film unleashes fresh wave of Bronte-mania
-
US backs Pakistan's 'right to defend itself' after strikes on Afghanistan

-
 Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP
Bezzecchi beats Marquez to pole at season-opening Thailand MotoGP
-
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic

-
 Oscar-nominated 'F1' sound engineers recreate roar of racetrack
Oscar-nominated 'F1' sound engineers recreate roar of racetrack
-
15 dead as cash-packed military plane crashes in Bolivia

-
 Costa Rica's Grynspan pledges reform in bid for UN chief job
Costa Rica's Grynspan pledges reform in bid for UN chief job
-
Former All Black Bridge hailed for influence at Western Force

-
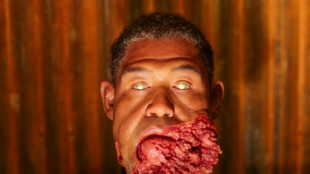 'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
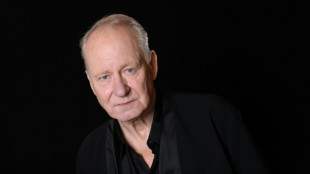
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
 'Brilliant industry' sees Reds down Highlanders in Super Rugby
'Brilliant industry' sees Reds down Highlanders in Super Rugby
-
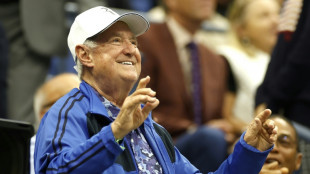
Neil Sedaka, US singer and songwriter, dies age 86
-
 Paramount acquires Warner Bros. in $110 bn mega-merger
Paramount acquires Warner Bros. in $110 bn mega-merger
-
Rosenior eyes extended stay to stabilise Chelsea

-
 Spurs struggling physically admits Tudor
Spurs struggling physically admits Tudor
-
Lens held by Strasbourg in blow to Ligue 1 title chances

-
 NFL salary cap passes $300 mn for first time
NFL salary cap passes $300 mn for first time
-
Wolves secure rare win to dent Villa's bid for Champions League place

-
 Oil prices jump on Iran attack fears while US stocks fall
Oil prices jump on Iran attack fears while US stocks fall
-
Two dead, dozens injured as tram derails in Milan

-
 Trump tells US govt to 'immediately' stop using Anthropic AI tech
Trump tells US govt to 'immediately' stop using Anthropic AI tech
-
Court orders Greenpeace to pay $345 mn to US oil pipeline company

-
 IAEA stresses 'urgency' to verify Iran's nuclear material
IAEA stresses 'urgency' to verify Iran's nuclear material
-
UN urges action to prevent full civil war in South Sudan

-
 Hackers steal medical details of 15 million in France
Hackers steal medical details of 15 million in France
-
Susan Sarandon praises Spain’s stance on Gaza

-
 Murray adamant size isn't everything despite losing Wales place
Murray adamant size isn't everything despite losing Wales place
-
Messi knocked down by fan in Puerto Rico pitch invasion

-
 Two killed, dozens injured as tram derails in Milan
Two killed, dozens injured as tram derails in Milan
-
O'Neill taken aback by Rangers boss Rohl's comments on Celtic

-
 Ukrainian, Slovak leaders hold call amid energy spat
Ukrainian, Slovak leaders hold call amid energy spat
-
French hard-left firebrand sparks row with 'antisemitic' Epstein jibe

-
 Ahmed, Jacks blast England to thrilling win over New Zealand
Ahmed, Jacks blast England to thrilling win over New Zealand
-
UK police arrest man after Churchill statue sprayed with graffiti

-
 Bill Clinton denies wrongdoing at grilling on Epstein ties
Bill Clinton denies wrongdoing at grilling on Epstein ties
-
Red Cross urges Afghanistan-Pakistan 'de-escalation'


IGC Pharma Expands CALMA Trial to Oklahoma, Partnering with Dr. David McCoy at Tekton Research in Yukon
POTOMAC, MD / ACCESS Newswire / June 23, 2025 / IGC Pharma, Inc. ("IGC", or the "Company") (NYSE American:IGC) today announced the addition of a new clinical trial site at Tekton Research in Yukon, Oklahoma, for its Phase 2 CALMA study evaluating IGC-AD1 for agitation in Alzheimer's dementia. This expansion into the Oklahoma City metropolitan area underscores IGC's commitment to diversifying its trial population and addressing regional healthcare disparities.
Tekton Research has extensive experience conducting clinical trials in therapeutic areas such as Alzheimer's disease, migraine, vaccines, and others. Dr. David McCoy, a board-certified neurologist in Canadian County and a native Oklahoman, will serve as the principal investigator at the Yukon site. Dr. McCoy plays a key role in his community by providing accessible healthcare and maintaining strong relationships with his patients.
"We are excited to collaborate with Dr. McCoy and Tekton Research to bring the CALMA trial to the Yukon community," said Ram Mukunda, CEO of IGC Pharma. "Expanding our trial to Oklahoma allows us to reach a broader and more diverse patient population, which is essential for the development of effective and inclusive treatments for Alzheimer's-related agitation."
Patients and caregivers interested in participating in the CALMA trial at the Yukon site can contact Tekton Research at 1804 Commons Circle, Suite B, Yukon, OK 73099, call (405) 594-7712, or email [email protected]. For more information, visit tektonresearch.com.
About IGC-AD1 and the CALMA Trial
IGC-AD1 is IGC Pharma's investigational cannabinoid-based therapy currently in a Phase 2 multicenter, double-blind, randomized, placebo-controlled study (CALMA) evaluating its safety and efficacy for treating agitation in Alzheimer's dementia. Agitation affects up to 76% of Alzheimer's patients, often leading to increased hospitalization and caregiver burden. IGC-AD1 acts as a partial CB1 receptor agonist with anti-neuroinflammatory properties, targeting key pathways involved in neuroinflammation, oxidative stress, and neurotransmitter imbalances.
For more information on the CALMA trial, visit: ClinicalTrials.gov.
About IGC Pharma (dba IGC):
IGC Pharma (NYSE American:IGC) is a clinical-stage biotechnology company leveraging AI to develop innovative treatments for Alzheimer's and metabolic disorders. Our lead asset, IGC-AD1, is a cannabinoid-based therapy currently in a Phase 2 trial (CALMA) for agitation in Alzheimer's dementia. Our pipeline includes TGR-63, targeting amyloid plaques, and early-stage programs focused on neurodegeneration, tau proteins, and metabolic dysfunctions. We integrate AI to accelerate drug discovery, optimize clinical trials, and enhance patient targeting. With 30 patent filings and a commitment to innovation, IGC Pharma is advancing breakthrough therapies.
About Tekton
Founded in 2006, Tekton Research is a multi-state clinical research site network conducting Phase 1-4 trials in CNS, cardiometabolic, general medicine and infectious disease. Led by seasoned professionals and nationally recognized KOLs, Tekton delivers scientific rigor and operational excellence across trials of any size. The company partners with sponsors, CROs, and biopharma to accelerate the development of new therapies while maintaining a strong commitment to patient-centered care.
Forward-Looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 24, 2024, and on Form 10-Qs filed with the SEC on August 7, 2024, November 12, 2024, and February 14, 2025, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur.
Contact Information
Rosalyn Christian/Walter Frank
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
View the original press release on ACCESS Newswire
P.Martin--AMWN


