
-
 OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
OpenAI strikes Pentagon deal with 'safeguards' as Trump dumps Anthropic
-
Oscar-nominated 'F1' sound engineers recreate roar of racetrack

-
 15 dead as cash-packed military plane crashes in Bolivia
15 dead as cash-packed military plane crashes in Bolivia
-
Costa Rica's Grynspan pledges reform in bid for UN chief job

-
 Former All Black Bridge hailed for influence at Western Force
Former All Black Bridge hailed for influence at Western Force
-
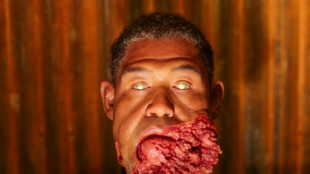
'Sinners' vampires inspired by animals, says Oscar hopeful makeup artist
-
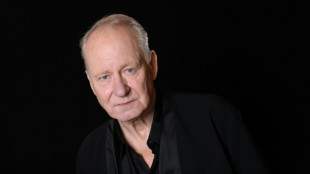 For Oscar nominee Stellan Skarsgard, good cinema is like slow food
For Oscar nominee Stellan Skarsgard, good cinema is like slow food
-
'Brilliant industry' sees Reds down Highlanders in Super Rugby

-
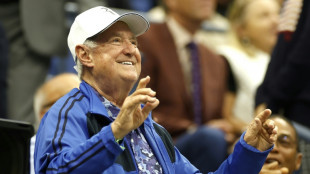 Neil Sedaka, US singer and songwriter, dies age 86
Neil Sedaka, US singer and songwriter, dies age 86
-
Paramount acquires Warner Bros. in $110 bn mega-merger

-
 Rosenior eyes extended stay to stabilise Chelsea
Rosenior eyes extended stay to stabilise Chelsea
-
Spurs struggling physically admits Tudor

-
 Lens held by Strasbourg in blow to Ligue 1 title chances
Lens held by Strasbourg in blow to Ligue 1 title chances
-
NFL salary cap passes $300 mn for first time

-
 Wolves secure rare win to dent Villa's bid for Champions League place
Wolves secure rare win to dent Villa's bid for Champions League place
-
Oil prices jump on Iran attack fears while US stocks fall

-
 Two dead, dozens injured as tram derails in Milan
Two dead, dozens injured as tram derails in Milan
-
Trump tells US govt to 'immediately' stop using Anthropic AI tech

-
 Court orders Greenpeace to pay $345 mn to US oil pipeline company
Court orders Greenpeace to pay $345 mn to US oil pipeline company
-
IAEA stresses 'urgency' to verify Iran's nuclear material

-
 UN urges action to prevent full civil war in South Sudan
UN urges action to prevent full civil war in South Sudan
-
Hackers steal medical details of 15 million in France

-
 Susan Sarandon praises Spain’s stance on Gaza
Susan Sarandon praises Spain’s stance on Gaza
-
Murray adamant size isn't everything despite losing Wales place

-
 Messi knocked down by fan in Puerto Rico pitch invasion
Messi knocked down by fan in Puerto Rico pitch invasion
-
Two killed, dozens injured as tram derails in Milan

-
 O'Neill taken aback by Rangers boss Rohl's comments on Celtic
O'Neill taken aback by Rangers boss Rohl's comments on Celtic
-
Ukrainian, Slovak leaders hold call amid energy spat

-
 French hard-left firebrand sparks row with 'antisemitic' Epstein jibe
French hard-left firebrand sparks row with 'antisemitic' Epstein jibe
-
Ahmed, Jacks blast England to thrilling win over New Zealand

-
 UK police arrest man after Churchill statue sprayed with graffiti
UK police arrest man after Churchill statue sprayed with graffiti
-
Bill Clinton denies wrongdoing at grilling on Epstein ties

-
 Red Cross urges Afghanistan-Pakistan 'de-escalation'
Red Cross urges Afghanistan-Pakistan 'de-escalation'
-
Coup role revelations revive calls for return of Spain's ex king

-
 Oil prices jump on Iran attack fears, Wall Street slips on AI
Oil prices jump on Iran attack fears, Wall Street slips on AI
-
TikTok disinformation: the other weapon in Mexico violence

-
 Carmaker BMW to trial humanoid robots at German factory
Carmaker BMW to trial humanoid robots at German factory
-
NASA announces overhaul of Artemis lunar program amid technical delays

-
 Golfer Pavan undergoes surgery after freak lift fall
Golfer Pavan undergoes surgery after freak lift fall
-
Bill Clinton faces grilling on extensive ties to Epstein

-
 For Roberto Cavalli designer, dreams come in all black
For Roberto Cavalli designer, dreams come in all black
-
Macron to set out how France's nuclear arms could protect Europe

-
 Spin-heavy England restrict New Zealand to 159-7 in Super Eights
Spin-heavy England restrict New Zealand to 159-7 in Super Eights
-
Starmer vows to fight 'extremes' after UK Labour election drubbing

-
 New Pokemon titles on horizon as 30th anniversary approaches
New Pokemon titles on horizon as 30th anniversary approaches
-
Arteta backs Gyokeres to impact Arsenal's trophy charge

-
 55 Ghanaians killed after being lured into Ukraine war: govt
55 Ghanaians killed after being lured into Ukraine war: govt
-
OpenAI raises $110 bn in record funding round

-
 Medvedev swats Auger-Aliassime aside to reach Dubai final
Medvedev swats Auger-Aliassime aside to reach Dubai final
-
Stocks slide, oil jumps tracking AI and Iran


Xenetic Biosciences, Inc. Announces Update from Collaboration Partner of First Patient Dosed in Exploratory Clinical Study of DNase I in Combination with FOLFIRINOX for the First Line Treatment of Unresectable, Locally Advanced or Metastatic Pancreatic Cancer
Investigator initiated exploratory clinical study being conducted in Israel pursuant to agreement with collaboration partner, PeriNess
Company evaluating systemic recombinant human DNase I (DNase I) in combination with chemotherapy and immunotherapy platforms for the treatment of pancreatic carcinoma, colorectal cancer and other locally advanced or metastatic solid tumors
FRAMINGHAM, MA / ACCESS Newswire / July 8, 2025 / Xenetic Biosciences, Inc. (NASDAQ:XBIO) ("Xenetic" or the "Company"), a biopharmaceutical company focused on advancing innovative immuno-oncology technologies addressing difficult to treat cancers, today announced that its collaboration partner, PeriNess Ltd. (PeriNess), has informed the Company that Bnei Zion Medical Center has commenced patient dosing in an exploratory clinical study of systemic DNase I in combination with FOLFIRINOX for the first line treatment of unresectable, locally advanced or metastatic pancreatic cancer.
Dr. Abed Agbabrya, head of Oncology at the Bnei Zion Hospital, will act as the principal investigator and all work will be conducted at The Fund for Medical Research, Development of Infrastructure and Health services - Bnei Zion Medical Center in Israel.
Dr. Dmitry Genkin, Xenetic Chairman stated, "We are very pleased with the update provided by PeriNess and for the start of patient dosing in this exploratory study. We remain committed to advancing our systemic recombinant human DNase I technology into the clinical stage. The ability of DNase I to degrade neutrophil extracellular traps (NETs) in the pancreatic cancer tumor microenvironment holds promise to improve clinical responses in a critically underserved patient population. We look forward to further exploring the full potential of DNase I."
PeriNess has informed the Company that the exploratory study is evaluating the safety, biomarker response, pharmacokinetics (PK) and clinical activity of DNase I in combination with first line regimen of FOLFIRINOX chemotherapy in patients with locally advanced or metastatic pancreatic cancer. All patients will receive intravenous infusions of DNase I on Days 1 and 8 of consecutive 14-day cycles. Safety will be continuously evaluated until the end of the study. DNase I pharmacokinetics will be evaluated on Days 1 and 2 of the first FOLFIRINOX cycle and Days 1 and 2 of the third FOLFIRINOX cycle. Neutrophil extracellular traps biomarkers will be evaluated on Day 1 of the first FOLFIRINOX cycle and every 4 weeks thereafter. Clinical activity will be evaluated by the Objective Response Rate (ORR) using Response Evaluation Criteria in Solid Tumors (RECIST 1.1) and Progression-Free Survival (PFS).
As previously announced, in December 2024, Xenetic entered into a Clinical Trial Services Agreement with PeriNess, under which PeriNess will lead in the regulatory approval, operational execution and management of potential exploratory, investigator initiated studies of recombinant DNase as an adjunctive treatment in patients with pancreatic carcinoma and other locally advanced or metastatic solid tumors receiving chemotherapy and immunotherapy in Israeli medical centers.
About Xenetic Biosciences
Xenetic Biosciences, Inc. is a biopharmaceutical company focused on advancing innovative immuno-oncology technologies addressing difficult to treat cancers. The Company's DNase technology is designed to improve outcomes of existing treatments, including immunotherapies, by targeting neutrophil extracellular traps (NETs), which are involved in the progression of many human cancers. Xenetic is currently focused on advancing its systemic DNase I program into the clinic as an adjunctive therapy for pancreatic carcinoma and locally advanced or metastatic solid tumors.
For more information, please visit the Company's website at www.xeneticbio.com and connect on X, LinkedIn, and Facebook.
Forward-Looking Statements
This press release contains forward-looking statements that we intend to be subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release other than statements of historical facts may constitute forward-looking statements within the meaning of the federal securities laws. These statements can be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," "remain," "focus", "confidence in", "potential", "look forward", "holds", and other words of similar meaning, including, but not limited to, all statements regarding expectations with respect to the investigator initiated exploratory study being conducted by Bnei Zion Medical Center evaluating the safety, biomarker response, PK and clinical activity of DNase I in combination with first line regimen of FOLFIRINOX chemotherapy in patients with locally advanced or metastatic pancreatic cancer, including the first patient dosing under the exploratory study; all statements regarding expectations with respect to our collaboration with PeriNess; and all statements regarding expectations for our DNase I-based oncology platform, including statements regarding: DNase holding promise to improve clinical responses in a critically underserved patient population, our expectations regarding exploring the full potential of DNase I, our focus on advancing innovative immune-oncology technologies addressing difficult to treat cancers, the DNase I technology improving outcomes of existing treatments, including immunotherapies, by targeting neutrophil extracellular traps (NETs), which are involved in the progression of many human cancers, and our focus on advancing our systemic DNase I program into the clinic as an adjunctive therapy for pancreatic carcinoma and locally advanced or metastatic solid tumors.
Any forward-looking statements contained herein are based on current expectations and are subject to a number of risks and uncertainties. Many factors could cause our actual activities, performance, achievements, or results to differ materially from the activities and results anticipated in forward-looking statements. Important factors that could cause actual activities, performance, achievements, or results to differ materially from such plans, estimates or expectations include, among others, (1) the relevance of, or our ability to utilize, the data from the investigator initiated exploratory study, if any, (2)) unexpected costs, charges or expenses resulting from our manufacturing and collaboration agreements, including the Clinical Trial Services Agreement with PeriNess; (3) unexpected costs, charges or expenses resulting from the licensing of the DNase platform; (4) uncertainty of the expected financial performance of the Company following the licensing of the DNase platform; (5) failure to realize the anticipated potential of the DNase technologies; (6) the ability of the Company to obtain funding and implement its business strategy; and (7) other risk factors as detailed from time to time in the Company's reports filed with the SEC, including its annual report on Form 10-K, periodic quarterly reports on Form 10-Q, current reports on Form 8-K and other documents filed with the SEC. The foregoing list of important factors is not exclusive. In addition, forward-looking statements may also be adversely affected by general market factors, general economic and business conditions, including potential adverse effects of public health issues and geopolitical events, such as the conflicts in the Ukraine and Middle East, on economic activity, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new product candidates and indications, manufacturing issues that may arise, patent positions, litigation, and shareholder activism, among other factors. The forward-looking statements contained in this press release speak only as of the date the statements were made, and the Company does not undertake any obligation to update forward-looking statements, except as required by law.
Contact:
JTC Team, LLC
Jenene Thomas
(908) 824-0775
[email protected]
SOURCE: Xenetic Biosciences, Inc.
View the original press release on ACCESS Newswire
M.Thompson--AMWN

