-
 Injury forces Marquez to adapt for MotoGP opener
Injury forces Marquez to adapt for MotoGP opener
-
Booming markets propel Hong Kong exchange's profits to record high

-
 West Indies recover from 83-7 to post to 176-8 against South Africa
West Indies recover from 83-7 to post to 176-8 against South Africa
-
Filmmakers defend Berlin festival chief in Gaza row

-
 Hong Kong mogul Jimmy Lai wins appeal in fraud case
Hong Kong mogul Jimmy Lai wins appeal in fraud case
-
Iranian in possible prisoner exchange faces 'terrorism' verdict in France

-
 'Street-smart' New Zealand can topple England to make T20 semis: coach
'Street-smart' New Zealand can topple England to make T20 semis: coach
-
Iran-US talks begin in push to avert war

-
 Merz says Germany, China must overcome trade gaps 'together'
Merz says Germany, China must overcome trade gaps 'together'
-
Automaker Stellantis posts massive loss, pivots from EV

-
 US, Ukraine to meet in Geneva after overnight Russian strikes
US, Ukraine to meet in Geneva after overnight Russian strikes
-
Snake-like robot unveiled for Fukushima debris removal

-
 'Public lynching': Senegal cracks down on LGBTQ+ community
'Public lynching': Senegal cracks down on LGBTQ+ community
-
Hong Kong sentences father of wanted activist to 8 months in jail

-
 The woman fighting to reclaim her face from Albania's 'AI minister'
The woman fighting to reclaim her face from Albania's 'AI minister'
-
Bulgaria ski station becomes refuge for digital nomads

-
 Thai runner-up party seeks criminal case against election officials
Thai runner-up party seeks criminal case against election officials
-
North Korea's Kim shuns South but could 'get along' with US

-
 Spurs win 10th straight, Pistons silence Thunder in battle of NBA's best
Spurs win 10th straight, Pistons silence Thunder in battle of NBA's best
-
Germany's Merz visits China AI hub hoping for business deals

-
 Post-uprising polls won't shake Nepal's delicate India-China balance
Post-uprising polls won't shake Nepal's delicate India-China balance
-
S.Korea's Park Chan-wook to head Cannes festival jury
-
 Australian ex-PM says 'more important than ever' to ditch UK monarchy
Australian ex-PM says 'more important than ever' to ditch UK monarchy
-
Dressed for succession? Kim Jong Un, daughter fuel speculation with matching coats

-
 US-Ukraine talks to open in Geneva after overnight Russian strikes
US-Ukraine talks to open in Geneva after overnight Russian strikes
-
Export ban sparks rush to process lithium in Zimbabwe

-
 Pakistani sculptor turns scrap into colossal metal artworks
Pakistani sculptor turns scrap into colossal metal artworks
-
Epstein files reveal links to cash, women, power in Africa

-
 Where are Southeast Asia's data centres?
Where are Southeast Asia's data centres?
-
Where AI lives: Southeast Asia's data centre boom

-
 Seoul hits fresh record on mixed day for Asia markets
Seoul hits fresh record on mixed day for Asia markets
-
Kyiv residents pool together for solar panels and batteries amid Russian strikes

-
 North Korea's Kim says could 'get along' with US but shuns South
North Korea's Kim says could 'get along' with US but shuns South
-
Cuba kills four on US-registered speedboat trying to 'infiltrate'

-
 UK Labour party threatened by hard-right, leftists in heartland
UK Labour party threatened by hard-right, leftists in heartland
-
Australian PM sorry after saying sexual assault survivor 'difficult'

-
 Kim Jong Un spurns olive branch from 'hostile' South Korea
Kim Jong Un spurns olive branch from 'hostile' South Korea
-
DR Congo sanctuary resists bloody forest sell-off

-
 North Korea looking to replicate youth success at Women's Asian Cup
North Korea looking to replicate youth success at Women's Asian Cup
-
Deal or no deal: What's the state of Trump's tariffs?

-
 Hillary Clinton to testify in US House panel's Epstein probe
Hillary Clinton to testify in US House panel's Epstein probe
-
African migrants won legal protections - then Trump deported them

-
 US women's ice hockey captain responds to 'distasteful' Trump remark
US women's ice hockey captain responds to 'distasteful' Trump remark
-
US presses missile issue as new Iran talks to open in Geneva

-
 US government accused of major 'cover-up' over Trump sex abuse claims
US government accused of major 'cover-up' over Trump sex abuse claims
-
US eases Cuba oil embargo but demands 'dramatic' change

-
 IMF urges US to work with partners to ease trade restrictions
IMF urges US to work with partners to ease trade restrictions
-
Brumbies not getting carried away by emphatic Super Rugby start

-
 Metallic Minerals Reports Successful Electrochemical Recovery of 99.9% Pure Copper Metal from La Plata Sulphide Mineralization
Metallic Minerals Reports Successful Electrochemical Recovery of 99.9% Pure Copper Metal from La Plata Sulphide Mineralization
-
Atha Energy Final Assays From 2025 Angilak Exploration Program Confirm High-Grade Discoveries at KU and Mushroom Lake Targets - Grades up to 1.56% U3O8 and 1.10% U3O8, Respectively, and Expansion of Mineralization at the Lac 50 Deposit With Grades up to 1.47% U3O8


Xenetic Biosciences, Inc. Announces Entry by Collaboration Partner into Clinical Study Agreement to Advance Development of DNase Platform for the Treatment of Large B Cell Lymphoma
Investigator initiated study with collaboration partner, PeriNess, to be conducted at the Tel-Aviv Sourasky Medical Center
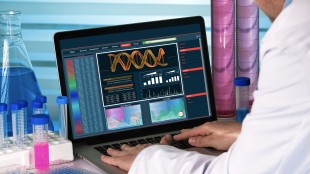
FRAMINGHAM, MA / ACCESS Newswire / July 30, 2025 / Xenetic Biosciences, Inc. (NASDAQ:XBIO) ("Xenetic" or the "Company"), a biopharmaceutical company focused on advancing innovative immuno-oncology technologies addressing difficult to treat cancers, today announced that its collaboration partner, PeriNess Ltd. ("PeriNess"), has entered into a Clinical Study Agreement (the "Agreement") to support an exploratory clinical study of DNase I in combination with anti-CD19 CAR T cells in patients with large B cell lymphoma.
Dr. Ron Ram, Professor of Medicine and Head of the Bone Marrow Transplantation Unit at the Tel Aviv Sourasky Medical Center ("Sourasky Center"), will act as the principal investigator of the study.
The primary objective of this study is to explore the safety and tolerability of DNase I in combination with anti-CD19 CAR T therapy in subjects with stable or progressive large B-cell lymphoma when DNase I is given in an adjuvant setting. Secondary objectives include efficacy to be evaluated by the measure of complete response rate post CAR T infusion, duration of response and overall survival. The study has the potential for a strong translational component with a complex assessment of biomarker response and analysis of anti-CD19 CAR T expansion and persistence.
"Our data suggests that the degradation of Neutrophil Extracellular Traps (NETs) by DNase I plays a crucial role in maintaining CAR T-cell function and preventing premature CAR T-cell exhaustion. Our preclinical studies conducted show that co-administration of DNase I with anti-CD19 CAR T cells significantly reduce tumor burden, delay tumor relapse and substantially prolong survival compared to the anti-CD19 CAR T cell monotherapy groups in various syngeneic and xenogeneic experimental models of lymphoma and leukemia," stated Alexey Stepanov, PhD, Institute Investigator at the Scripps Research Institute, and a member of Xenetic's Scientific Steering Committee.
"Progression of large B cell lymphoma (LBCL) is the major obstacle for the success of CAR T therapies, with approximately 40-60% of the patients relapsing in the first year, and 25-35% within 3 months after CAR T infusion, depending on the CAR T product used. While patients with partial or complete response before CAR T infusion have a 1-year progression free survival of 60-80%, those with stable or progressive disease at the time of CAR T infusion have a 1-year progression free survival of 20-30%. NETs facilitate several hallmarks of cancer biology at various stages, including progression, invasion, metastasis, immunosuppression, immune escape, and resistance to therapy. A high content of NETs in lymphoma tissue and blood of patients was associated with a negative outcome. The goal of this clinical study is to improve clinical response by administering DNase I to abrogate the negative effects of NETs on the performance of immune system and CAR T cells," added Dr. Ram.
James Parslow, Interim Chief Executive Officer and Chief Financial Officer of Xenetic concluded, "We are pleased with the continued progress of our DNase I program and the expansion of its development in another exploratory study to further evaluate its potential in various oncology indications. We look forward to garnering additional data to realize the full potential of DNase I."
As previously announced, in December 2024, Xenetic entered into a Clinical Trial Services Agreement with PeriNess, under which PeriNess will lead in the regulatory approval, operational execution and management of potential exploratory, investigator-initiated studies of recombinant DNase I as an adjunctive treatment in patients with pancreatic carcinoma and other locally advanced or metastatic solid tumors receiving chemotherapy and immunotherapy in Israeli medical centers.
About Xenetic Biosciences
Xenetic Biosciences, Inc. is a biopharmaceutical company focused on advancing innovative immuno-oncology technologies addressing difficult to treat cancers. The Company's DNase technology is designed to improve outcomes of existing treatments, including immunotherapies, by targeting neutrophil extracellular traps (NETs), which are involved in the progression of many human cancers. Xenetic is currently focused on advancing its systemic DNase I program into the clinic as an adjunctive therapy for pancreatic carcinoma and locally advanced or metastatic solid tumors.
For more information, please visit the Company's website at www.xeneticbio.com and connect on X, LinkedIn, and Facebook.
Forward-Looking Statements
This press release contains forward-looking statements that we intend to be subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release other than statements of historical facts may constitute forward-looking statements within the meaning of the federal securities laws. These statements can be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," "remain," "focus", "confidence in", "potential", and other words of similar meaning, including, but not limited to, all statements regarding expectations with respect to the Clinical Trial Services Agreement with PeriNess, including statements regarding the proposed investigator-initiated study under such agreement to support an exploratory clinical study of our systemic DNase I candidate in patients with large B-cell lymphoma and the expected objectives and goal of such study, and all statements regarding expectations for our DNase-base oncology platform, including statements regarding: our overall development strategy, the progress of our DNase I program, our expectations regarding further expansion of our body of clinical data, our focus on advancing innovative immune-oncology technologies addressing difficult to treat cancers, the DNase technology improving outcomes of existing treatments, including immunotherapies, by targeting neutrophil extracellular traps (NETs), which are involved in the progression of many cancers, and our focus on advancing our systemic DNase program into the clinic as an adjunctive therapy for pancreatic carcinoma and locally advanced or metastatic solid tumors. Any forward-looking statements contained herein are based on current expectations and are subject to a number of risks and uncertainties. Many factors could cause our actual activities, performance, achievements, or results to differ materially from the activities and results anticipated in forward-looking statements. Important factors that could cause actual activities, performance, achievements, or results to differ materially from such plans, estimates or expectations include, among others, (1) the relevance of, or our ability to utilize, the data, if any, from any investigator-initiated exploratory study, (2) unexpected costs, charges or expenses resulting from our manufacturing and collaboration agreements, including the Clinical Trial Services Agreement with PeriNess; (3) unexpected costs, charges or expenses resulting from the licensing of the DNase platform; (4) uncertainty of the expected financial performance of the Company following the licensing of the DNase platform; (5) failure to realize the anticipated potential of the DNase technologies; (6) the ability of the Company to obtain funding and implement its business strategy; and (7) other risk factors as detailed from time to time in the Company's reports filed with the SEC, including its annual report on Form 10-K, periodic quarterly reports on Form 10-Q, current reports on Form 8-K and other documents filed with the SEC. The foregoing list of important factors is not exclusive. In addition, forward-looking statements may also be adversely affected by general market factors, general economic and business conditions, including potential adverse effects of public health issues and geopolitical events, such as the conflicts in the Ukraine and in the Middle East, on economic activity, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new product candidates and indications, manufacturing issues that may arise, patent positions, litigation, and shareholder activism, among other factors. The forward-looking statements contained in this press release speak only as of the date the statements were made, and the Company does not undertake any obligation to update forward-looking statements, except as required by law.
Contact:
JTC Team, LLC
Jenene Thomas
(908) 824-0775
[email protected]
SOURCE: Xenetic Biosciences, Inc.
View the original press release on ACCESS Newswire
P.Santos--AMWN