-
 Inside the bunker where Zelensky led response to Russian invasion
Inside the bunker where Zelensky led response to Russian invasion
-
France demands explanation from US envoy over 'surprise' no-show

-
 Putin failed to achieve goals in Ukraine, Zelensky says on war anniversary
Putin failed to achieve goals in Ukraine, Zelensky says on war anniversary
-
China tightens Japanese trade restrictions as spat worsens

-
 Ukraine war exhibition opens at Berlin Nazi bunker museum
Ukraine war exhibition opens at Berlin Nazi bunker museum
-
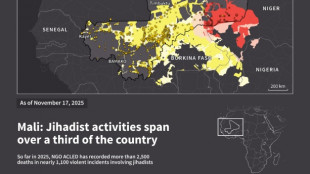
Jihadist threat puts eastern Senegal on edge
-
 Kim Yo Jong: the powerful sister behind North Korea's supreme leader
Kim Yo Jong: the powerful sister behind North Korea's supreme leader
-
North Korea ruling party promotes Kim Jong Un's younger sister

-
 Mexico's Jalisco cautiously tries returning to normal after cartel violence
Mexico's Jalisco cautiously tries returning to normal after cartel violence
-
Mexico's violence-hit Guadalajara to host World Cup games

-
 Mourinho's Bernabeu homecoming upended by suspension, racism row
Mourinho's Bernabeu homecoming upended by suspension, racism row
-
China targets Japanese companies over military ties

-
 Griezmann in talks to join MLS side Orlando City: source
Griezmann in talks to join MLS side Orlando City: source
-
France to revoke US envoy's govt access after summons no-show

-
 Spurs overpower Pistons in clash of NBA's form teams
Spurs overpower Pistons in clash of NBA's form teams
-
Inoue to fight Nakatani in Tokyo in May: reports

-
 Canada PM to push trade, rebuild fractured ties in India trip
Canada PM to push trade, rebuild fractured ties in India trip
-
Asian markets mixed as traders weigh AI and tariffs outlook
-
 Votes may 'melt like snow': Reform, Greens eye Labour UK bastion
Votes may 'melt like snow': Reform, Greens eye Labour UK bastion
-
Venezuela says exiles welcome to return following mass amnesty

-
 Australia buys parts for future AUKUS sub reactor
Australia buys parts for future AUKUS sub reactor
-
Ukraine marks four years since Russian invasion

-
 Brazil court to try politicians over hit on black councilwoman
Brazil court to try politicians over hit on black councilwoman
-
Interim president says Venezuelans welcome to return after amnesty law

-
 Man kills police officer in Moscow train station blast
Man kills police officer in Moscow train station blast
-
Despite drop in 2025, Russian oil exports exceed pre-war volumes: report

-
 Bytek Joins the Google Cloud Ready - BigQuery Program
Bytek Joins the Google Cloud Ready - BigQuery Program
-
Formation Metals Intersects 0.95 g/t Au over 61.1 Metres, including 1.68 g/t Au over 26.5 Metres at the Advanced N2 Gold Project; Bulk-Tonnage Gold Target Identified with 8 Kilometres of Strike to Explore

-
 Bolt Metals Announces Closing of Fully Subscribed Private Placement
Bolt Metals Announces Closing of Fully Subscribed Private Placement
-
InterContinental Hotels Group PLC Announces Transaction in Own Shares - February 24

-
 Nikon Expands Popular Monarch and Prostaff Binocular Lines
Nikon Expands Popular Monarch and Prostaff Binocular Lines
-
Australian PM seeks removal of UK's Andrew from line of succession

-
 Carrick hails 'ruthless' Man Utd match-winner Sesko
Carrick hails 'ruthless' Man Utd match-winner Sesko
-
N.Korea leader's sister promoted at party congress

-
 The key to taking down Mexico's most-wanted narco? His girlfriend
The key to taking down Mexico's most-wanted narco? His girlfriend
-
Winter storm blankets US northeast as travel bans imposed

-
 Super-sub Sesko fires Man Utd to win at Everton
Super-sub Sesko fires Man Utd to win at Everton
-
YouTube exec says goal was viewer value not addiction

-
 Panama wrests control of canal ports from Hong Kong group
Panama wrests control of canal ports from Hong Kong group
-
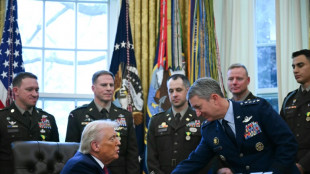
Trump denies top US officer warned of Iran strike risks
-
 Mayweather to fight Pacquiao in Las Vegas in September
Mayweather to fight Pacquiao in Las Vegas in September
-
US stocks tumble on tariff fog, worries over AI

-
 US says China 'massively expanded' nuclear arsenal
US says China 'massively expanded' nuclear arsenal
-
US forces to complete withdrawal from Syria within a month

-
 US winter storm brings rare hush to snowy New York
US winter storm brings rare hush to snowy New York
-
George adamant Six Nations losses don't make England 'a bad team overnight'

-
 US Supreme Court to hear bid to block climate change suits
US Supreme Court to hear bid to block climate change suits
-
Canada summons OpenAI over failure to report mass shooter

-
 From Odesa to Bakhmut, revisiting a Ukrainian family torn by war
From Odesa to Bakhmut, revisiting a Ukrainian family torn by war
-
Vonn says Olympic injury could have led to amputation


ZEO ScientifiX(TM) Positioned for New Growth as Florida Opens Stem Cell Market
New Florida stem cell law provides ZEO with new pathways to market and sell regenerative therapy products and advance clinical research initiatives
FORT LAUDERDALE, FL / ACCESS Newswire / October 23, 2025 / ZEO ScientifiX™, Inc. (OTCQB:ZEOX) ("ZEO" or the "Company"), a clinical‑stage biopharmaceutical company focused on regenerative therapies including birth tissue biologics, whole blood processing, and exosome nanoparticle science, today announced that it is prepared to capitalize on the potentially rapid expansion of Florida's regenerative medicine market following enactment of the new state stem cell law, SB 1768, effective July 1, 2025.
The legislation authorizes licensed physicians to administer non‑FDA‑approved stem cell and other human tissue‑derived therapies for orthopedic, wound care, and pain management indications. It mandates compliance with the FDA's current good manufacturing practices (cGMP) and prohibits the use of stem cells derived from aborted fetuses, promoting ethically sourced materials such as adult stem cells and umbilical cord blood. SB 1768 also requires informed patient consent and disclosure that treatments are not FDA‑approved.
ZEO expects that SB 1768 will create substantial new demand for its current and future stem cell therapy products by: (a) meeting existing physician and patient interest in regenerative therapies, (b) increasing awareness of the availability and potential efficacy of stem cell therapy treatments, and (c) attracting medical tourists who previously sought stem cell therapy treatments abroad but can now access comparable procedures in Florida under higher regulatory standards and at lower cost.
By enabling physicians to adopt ZEO's products for approved indications in Florida, the Company anticipates significant revenue growth and faster advancement of its clinical trial objectives at reduced cost. ZEO expects to further distinguish itself through leadership in research, quality, safety, and regulatory compliance for biologics. The new law also allows ZEO to collect real‑world safety and outcome data from providers-data that previously could only be obtained through FDA Investigational New Drug (IND) applications or Institutional Review Board (IRB)‑approved studies. Access to this data is expected to lower risks associated with the Company's future FDA submissions for product approvals.
While prioritizing the anticipated growth of Florida's stem cell market, ZEO continues to advance its research programs, including clinical studies, product development, and ongoing quality and compliance initiatives. The Company recently launched an IRB‑approved clinical study titled "Open‑Label Prospective Longitudinal Clinical Trial to Evaluate the Safety and Potential Efficacy of PPX™ in Patients Suffering from Musculoskeletal Joint Pathologies Using Real‑World Data to Monitor Patient Outcomes." The study will enroll up to 350 patients, with five clinics already participating and seven patients enrolled to date.
ZEO is also evaluating expansion of its laboratory facilities at Nova Southeastern University to include additional clean rooms and dedicated research space. These upgrades will support efforts to grow its intellectual property portfolio, expand provider networks, and increase product and clinical trial capacity.
"We believe ZEO is a trusted supplier of biologics, and our research continues to lead the industry," said Ian Bothwell, interim chief executive officer. "We look forward to announcing additional developments in the near future."
About ZEO ScientifiX™, Inc.
ZEO ScientifiX™, Inc. (OTCQB:ZEOX) is a clinical-stage biopharmaceutical company located at Nova Southeastern University's Collaborative Center for Research. Our proprietary products, including (a) Zofin™, which are derived from perinatal sources and manufactured to retain the naturally occurring extracellular vesicles, proteins and cell secreted nanoparticles, and (b) Patient Pure X™ ("PPX™"), an autologous biologic containing a nanoparticle fraction that is precipitated from a patient's own peripheral blood, are manufactured in an FDA-registered, cGMP-compliant laboratory. To learn more, please visit https://zeoscientifix.com.
Forward-Looking Statements
Certain statements contained in this press release should be considered forward-looking statements within the meaning of the Securities Act of 1933, as amended (the "Securities Act"), the Securities Exchange Act of 1934, as amended (the "Exchange Act"), and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are often identified by the use of forward-looking terminology such as "will," "believes," "expects," "potential," or similar expressions, involving known and unknown risks and uncertainties. Although the Company believes that the expectations reflected in these forward-looking statements are reasonable, they do involve assumptions, risks, and uncertainties, and these expectations may prove to be incorrect. No assurances can be given that the Florida Stem Cell Law will be beneficial to the Company or any initiatives related to this new law will increase our revenues and/or the price of our common stock to a level that is attractive to brokerage houses and institutional investors. We remind you that actual results could vary dramatically as a result of known and unknown risks and uncertainties, including but not limited to: potential issues related to our financial condition, competition, the ability to retain key personnel, product safety, efficacy and acceptance, the commercial success of any new products or technologies, success of clinical programs, ability to retain key customers, our inability to expand sales and distribution channels, legislation or regulations affecting our operations, including product pricing, reimbursement or access, the ability to protect our patents and other intellectual property both domestically and internationally, and other known and unknown risks and uncertainties, including the risk factors discussed in the Company's periodic reports that are filed with the SEC and available on the SEC's website (http://www.sec.gov). You are cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements attributable to the Company or persons acting on its behalf are expressly qualified in their entirety by these risk factors. Specific information included in this press release may change over time and may or may not be accurate after the date of the release. ZEO has no intention and specifically disclaims any duty to update the information in this press release.
Contact Information
ZEO Investor Relations
Jacqueline Domenech
1-888-963-7881
[email protected]
SOURCE: ZEO ScientifiX, Inc.
View the original press release on ACCESS Newswire
Ch.Kahalev--AMWN