
-
 Iran hangs three more accused of spying as fears grow for Swede
Iran hangs three more accused of spying as fears grow for Swede
-
Australia choose to bat first in first Test against West Indies

-
 Gambhir backs India bowlers to 'deliver' despite first Test misery
Gambhir backs India bowlers to 'deliver' despite first Test misery
-
Trump reassures allies as NATO agrees 'historic' spending hike

-
 England's Duckett says mindset change behind Test success
England's Duckett says mindset change behind Test success
-
Trump sees 'progress' on Gaza, raising hopes for ceasefire

-
 UK's Glastonbury Festival opens gates amid Kneecap controversy
UK's Glastonbury Festival opens gates amid Kneecap controversy
-
Oil rebounds as markets track Iran-Israel ceasefire

-
 Cable theft in north France disrupts Eurostar traffic
Cable theft in north France disrupts Eurostar traffic
-
Cambodians at quiet Thai border plead for peace

-
 Trump plays nice as NATO eyes 'historic' spending hike
Trump plays nice as NATO eyes 'historic' spending hike
-
Barcelona announce Camp Nou return for August 10

-
 Trump insists Iran nuclear programme set back 'decades'
Trump insists Iran nuclear programme set back 'decades'
-
Armenia PM says foiled 'sinister' coup plot by senior cleric

-
 Turkey breathes easier as Iran-Israel truce eases fallout risk
Turkey breathes easier as Iran-Israel truce eases fallout risk
-
Tesla sales skid in Europe in May despite EV rebound

-
 'Not Test class': Pundits tear into India after England chase 371
'Not Test class': Pundits tear into India after England chase 371
-
Trump whirlwind tests NATO summit unity

-
 Justice orders release of migrants deported to Costa Rica by Trump
Justice orders release of migrants deported to Costa Rica by Trump
-
Vietnam tycoon will not face death penalty over $27 bn fraud: lawyer

-
 Vietnam abolishes death penalty for spying, anti-state activities
Vietnam abolishes death penalty for spying, anti-state activities
-
Over 80,000 people flee severe flooding in southwest China

-
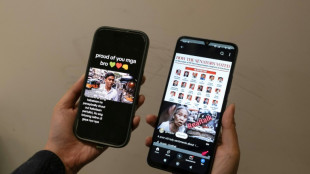 AI fakes duel over Sara Duterte impeachment in Philippines
AI fakes duel over Sara Duterte impeachment in Philippines
-
UK carbon emissions cut by half since 1990: experts

-
 Delap off mark as Chelsea ease into Club World Cup last 16
Delap off mark as Chelsea ease into Club World Cup last 16
-
UK to reintroduce nuclear weapon-capable aircraft under NATO

-
 Upstart socialist stuns political veteran in NYC mayoral primary
Upstart socialist stuns political veteran in NYC mayoral primary
-
China's premier warns global trade tensions 'intensifying'

-
 Chelsea through to Club World Cup knockouts, Benfica beat Bayern
Chelsea through to Club World Cup knockouts, Benfica beat Bayern
-
Cummins says Green 'long-term option' as Australia face new-look Windies

-
 Chelsea east past Esperance and into Club World Cup last 16
Chelsea east past Esperance and into Club World Cup last 16
-
Stocks rally as Iran-Israel ceasefire holds, oil claws back some losses

-
 Trump whirlwind to test NATO summit unity
Trump whirlwind to test NATO summit unity
-
Israel claims victory as US intel says Iran nuclear sites not destroyed

-
 Benfica beat Bayern at Club World Cup as Auckland City hold Boca
Benfica beat Bayern at Club World Cup as Auckland City hold Boca
-
RFK Jr's medical panel to revisit debunked vaccine claims

-
 Sean Combs trial: Takeaways from testimony
Sean Combs trial: Takeaways from testimony
-
Messi and Miami relishing reunion with PSG and Enrique

-
 At least 10 dead in Colombia landslide
At least 10 dead in Colombia landslide
-
Extreme heat, storms take toll at Club World Cup

-
 France's Versailles unveils AI-powered talking statues
France's Versailles unveils AI-powered talking statues
-
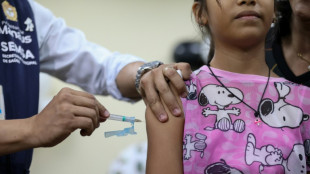
Child vaccine coverage faltering, threatening millions: study
-
 Fineqia Appoints Tether Co-Founder Reeve Collins to Advisory Board
Fineqia Appoints Tether Co-Founder Reeve Collins to Advisory Board
-
Petros Pharmaceuticals Announces Deconsolidation of Metuchen Pharmaceuticals Subsidiary, Strengthening its Balance Sheet and Improving the Company's Equity Position

-
 Fonon Technologies Introduces Heavy-Duty Stationary Laser Marking Machine for Military Components
Fonon Technologies Introduces Heavy-Duty Stationary Laser Marking Machine for Military Components
-
Northern Superior Announces Closing of $5 Million Private Placement and Welcomes NQ Investissement Minier as a Shareholder

-
 Preservica and Gravity Union Partner to Streamline Long-Term Data Archiving and Governance in Microsoft 365 for Canadian Organizations
Preservica and Gravity Union Partner to Streamline Long-Term Data Archiving and Governance in Microsoft 365 for Canadian Organizations
-
Jaguar Health to Pursue Approval of Canalevia in European Union for Treatment of General Diarrhea in Dogs

-
 Actress and Influencer Moran Atias Joins Saffron Tech as Brand Ambassador and Investor
Actress and Influencer Moran Atias Joins Saffron Tech as Brand Ambassador and Investor
-
Nocera, Inc. Makes Strategic Investment in Tachyonext Inc. to Enter U.S. E-Commerce Market and Expand Consumer Tech Portfolio


CNS Pharmaceuticals Acquires Orphan Drug Designation for Novel, Late Stage Abeotaxane, TPI 287
Company advancing plans to develop TPI 287, a novel, late stage abeotaxane that appears to cross the blood-brain barrier and has published clinical efficacy data in glioblastoma multiforme (GBM)
Management releases "What This Means" segment discussing the receipt of Orphan Drug Designations for TPI 287; Access here
HOUSTON, TX / ACCESS Newswire / May 13, 2025 / CNS Pharmaceuticals, Inc. (NASDAQ:CNSP) ("CNS" or the "Company"), a biopharmaceutical company specializing in the development of novel treatments for primary and metastatic cancers in the brain and central nervous system, today announced the successful transfer from Cortice Biosciences, Inc. of Orphan Drug Designation for TPI 287. The U.S. Food and Drug Administration (FDA) previously granted Orphan Drug Designations for TPI 287 in treating gliomas, pediatric neuroblastoma, and progressive supranuclear palsy. Additionally, the Company announced the release of a "What This Means" segment to discuss the Orphan Drug Designations for TPI 287, which is now available here.
TPI 287 is an abeotaxane with the same mechanism of action as other taxanes, such as paclitaxel (Taxol®) and docetaxel; it stabilizes microtubules and inhibits cell division, causing apoptosis and cell death. Although most taxanes are substrates for multi-drug resistant transporters that maintain the blood brain barrier (BBB), TPI 287's clinical data suggest it has the potential to cross the BBB and treat CNS tumors. In a Phase 1 trial treating glioblastoma patients with TPI 287 in combination with bevacizumab (Avastin®), data include 3 Complete Responses and 9 Partial Responses out of 23 patients evaluable.
CNS CEO John Climaco stated, "The successful transfer of the Orphan Drug Designations for TPI 287 demonstrates our operational efficiency as well as our ongoing commitment to the development of neuro oncology-focused, cutting-edge chemotherapies. As we prepare to commence patient enrollment for a Phase 2 study around year-end 2025, this important step also recognizes the significant protection Orphan status may confer in the form of seven years of market exclusivity. Our TPI 287 program is supported by published clinical and radiologic data showing positive activity against GBM, and a favorable safety profile from testing in hundreds of patients. With our demonstrated commitment to defeating this deadly disease, we will be aggressively developing TPI 287 as a treatment option for GBM, where patients continue to face a near-uniformly fatal unmet clinical need."
The FDA Office of Orphan Products Development grants Orphan Drug Designation to products that show the potential to treat rare diseases or conditions, which primarily are those that affect fewer than 200,000 people in the U.S. Orphan Drug Designation qualifies a company for a number of benefits intended to provide incentives to develop drugs for rare diseases or conditions, including tax credits, exemptions from certain FDA fees (which can be significant), and the potential for seven years of market exclusivity following drug approval.
About CNS Pharmaceuticals, Inc.
CNS Pharmaceuticals is a clinical-stage pharmaceutical company developing a pipeline of anti-cancer drug candidates for the treatment of primary and metastatic cancers of the brain and central nervous system.
The Company's drug candidate TPI 287 is an abeotaxane, which stabilizes microtubules and inhibits cell division, causing apoptosis and cell death. The initial clinical efficacy data suggest TPI 287 has the potential to cross the blood-brain barrier and treat CNS tumors. TPI 287 also has been tested in over 350 patients in clinical trials as a monotherapy and in combination with bevacizumab for the treatment of a range of diseases or conditions, including recurrent glioblastoma, recurrent neuroblastoma and medulloblastoma, advanced malignancies, progressive neoplastic disease, advanced unresectable pancreatic cancer, metastatic melanoma, and breast cancer metastatic to the brain. To date TPI 287 appears have both an excellent safety profile and high tolerability among patients.
For more information, please visit www.CNSPharma.com, and connect with the Company on X, Facebook, and LinkedIn.
Forward-Looking Statements
Some of the statements in this press release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the timing of the commencement of the Phase 2 study of TPI 287. These statements relate to future events, future expectations, plans and prospects. Although CNS believes the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. CNS has attempted to identify forward-looking statements by terminology including ''believes,'' ''estimates,'' ''anticipates,'' ''expects,'' ''plans,'' ''projects,'' ''intends,'' ''potential,'' ''may,'' ''could,'' ''might,'' ''will,'' ''should,'' ''approximately'' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including market and other conditions and those discussed under Item 1A. "Risk Factors" in CNS's most recently filed Form 10-K filed with the Securities and Exchange Commission ("SEC") and updated from time to time in its Form 10-Q filings and in its other public filings with the SEC. Any forward-looking statements contained in this press release speak only as of its date. CNS undertakes no obligation to update any forward-looking statements contained in this press release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events, except as required by law.
CONTACTS:
Investor Relations Contact
JTC Team, LLC
Jenene Thomas
908.824.0775
[email protected]
SOURCE: CNS Pharmaceuticals, Inc.
View the original press release on ACCESS Newswire
O.Karlsson--AMWN

