
-
 It will be 'big and punchy': Athletics chief Coe looks to future
It will be 'big and punchy': Athletics chief Coe looks to future
-
India's Pant reprimanded for dissent in first Test

-
 Oil prices drop as Israel agrees to ceasefire proposal
Oil prices drop as Israel agrees to ceasefire proposal
-
UK aims to tackle Google dominance of online search

-
 'Not at the level': Atletico left to ruminate after Club World Cup KO
'Not at the level': Atletico left to ruminate after Club World Cup KO
-
Border confusion as Thailand shuts land crossings with Cambodia

-
 Vietnam puts 41 on trial in $45 mn corruption case
Vietnam puts 41 on trial in $45 mn corruption case
-
World facing 'most complex' situation in decades: WEF

-
 Trial of Sean Combs approaches final stretch
Trial of Sean Combs approaches final stretch
-
Panama says has regained 'control' of restive province after months of protests

-
 Trump says Iran-Israel ceasefire in force
Trump says Iran-Israel ceasefire in force
-
Pharrell bigs up brown denim as Paris fashion week starts

-
 'Companions' ease pain of China's bustling, bamboozling hospitals
'Companions' ease pain of China's bustling, bamboozling hospitals
-
Japan PM to face tough upper house election on July 20

-
 Judge tells Australian mushroom murder jury to put emotion aside
Judge tells Australian mushroom murder jury to put emotion aside
-
Israel says 3 killed in Iran strike after Trump's ceasefire announcement

-
 Messi's Miami and PSG progress to set up Club World Cup reunion
Messi's Miami and PSG progress to set up Club World Cup reunion
-
Rock on: how crushed stone could help fight climate change

-
 Porto, Al Ahly out after sharing eight goals in thriller
Porto, Al Ahly out after sharing eight goals in thriller
-
Glamour, gripes as celebs head to Venice for exclusive Bezos wedding

-
 Messi to face PSG after Miami and Palmeiras draw to go through
Messi to face PSG after Miami and Palmeiras draw to go through
-
Schmidt warned he must release Wallabies for Lions warm-ups

-
 Palmeiras fight back against Inter Miami - both teams through
Palmeiras fight back against Inter Miami - both teams through
-
With missiles overhead, Tel Aviv residents huddle underground

-
 Virgin Australia surges in market comeback
Virgin Australia surges in market comeback
-
Asian stocks up as Trump announces Iran-Israel ceasefire

-
 Flatterer-in-chief: How NATO's Rutte worked to win over Trump
Flatterer-in-chief: How NATO's Rutte worked to win over Trump
-
Iran signals halt to strikes if Israel stops

-
 NATO summit seeks to keep Trump happy -- and alliance united
NATO summit seeks to keep Trump happy -- and alliance united
-
Russian drone attacks kill three in northeast Ukraine

-
 Better than gold: how Ecuador cashed in on surging cocoa prices
Better than gold: how Ecuador cashed in on surging cocoa prices
-
Millions in US sweat out first extreme heat wave of year

-
 Pro-Palestinian protest leader details 104 days spent in US custody
Pro-Palestinian protest leader details 104 days spent in US custody
-
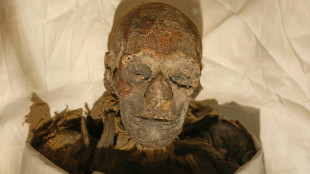
Gender not main factor in attacks on Egyptian woman pharaoh: study
-
 'Throwing the book away' with no preparation for next season: Bayern's Kompany
'Throwing the book away' with no preparation for next season: Bayern's Kompany
-
Global Financial Institutions and Technology Leaders Collaborate Under FINOS to Launch Open Source Common Controls for AI Services
-
 FILMSCAPE 2025 to Feature Interactive Instruction Using Global RGB LED Technologies' Newest Virtual Production Screens
FILMSCAPE 2025 to Feature Interactive Instruction Using Global RGB LED Technologies' Newest Virtual Production Screens
-
Star Copper Expedites Sample Shipments from Drill Campaign

-
 PathPresenter Receives FDA 510(k) Clearance for Digital Pathology Clinical Viewer
PathPresenter Receives FDA 510(k) Clearance for Digital Pathology Clinical Viewer
-
Amazing AI PLC Announces Bitcoin Treasury Policy

-
 Trump announces ceasefire between Iran and Israel
Trump announces ceasefire between Iran and Israel
-
US Supreme Court allows third country deportations to resume

-
 Oil prices tumble as markets shrug off Iranian rebuttal to US
Oil prices tumble as markets shrug off Iranian rebuttal to US
-
Rishabh Pant: India's unorthodox hero with 'method to his madness'

-
 PSG ease past Seattle Sounders and into Club World Cup last 16
PSG ease past Seattle Sounders and into Club World Cup last 16
-
Atletico win in vain as Botafogo advance at Club World Cup

-
 Osaka, Azarenka advance on grass at Bad Homburg
Osaka, Azarenka advance on grass at Bad Homburg
-
Haliburton latest NBA star with severe injury in playoffs

-
 Trump wants quick win in Iran, but goal remains elusive
Trump wants quick win in Iran, but goal remains elusive
-
Iran attacks US base in Qatar, Trump says time to make peace


CASI Pharmaceuticals Provides Business and Clinical Update
BEIJING, CHINA / ACCESS Newswire / May 19, 2025 / CASI Pharmaceuticals, Inc. (Nasdaq:CASI), ("CASI" or the "Company"), a Cayman incorporated biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products, announced that it will host a live conference call and webcast at 8:00 a.m. PT/11:00 a.m. ET on Wednesday, May 21, 2025 to provide business and clinical update.
A registration link and live webcast of the call is available below:
https://www.webcaster4.com/Webcast/Page/3120/52521
The presentation materials will be available in the Investors section of CASI's website after the conference call.
Further information regarding the Company can be found at www.casipharmaceuticals.com.
About CASI Pharmaceuticals
CASI Pharmaceuticals, Inc. is a biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products in China, the United States, and throughout the world. The Company is focused on acquiring, developing, and commercializing products that augment its focus on hematology oncology therapeutics and therapeutics for organ transplant rejection and autoimmune disease, as well as other areas of unmet medical need. The Company intends to execute its plan to become a leader by launching medicines in the Greater China market, leveraging the Company's China-based regulatory and commercial competencies and its global drug development expertise. The Company's operations in China are conducted through its wholly owned subsidiary, CASI Pharmaceuticals (China) Co., Ltd., located in Beijing, China. More information on CASI is available at www.casipharmaceuticals.com.
CASI Forward-Looking Statements
This announcement contains forward-looking statements. These statements are made under the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as "will," "expects," "anticipates," "future," "intends," "plans," "believes," "estimates," "confident" and similar statements. Among other things, the business outlook and quotations from management in this announcement, as well as the Company's strategic and operational plans, contain forward-looking statements. The Company may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the "SEC"), in its annual report to shareholders, in press releases and other written materials and in oral statements made by its officers, directors or employees to third parties. Statements that are not historical facts, including statements about the Company's beliefs and expectations, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties. A number of factors could cause actual results to differ materially from those contained in any forward-looking statement, including but not limited to the following: uncertainties related to the possibility that the Transaction will not occur as planned if events arise that result in the termination of the Equity and Assets Transfer Agreement, or if one or more of the various closing conditions to the Transaction are not satisfied or waived; the possibility that our plan with respect to our business operations after the consummation of the Transaction can be implemented successfully; our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern; the possibility that we may be delisted from trading on The Nasdaq Capital Market if we fail to satisfy applicable continued listing standards; the volatility in the market price of our ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict when or if our product candidates will be approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, PRC National Medical Products Administration, or other regulatory authorities; our inability to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products; and our dependence on third parties; the risks related to our dependence on Juventas to conduct the clinical development of CNCT19 and to partner with us to co-market CNCT19; risks related to our dependence on Juventas to ensure the patent protection and prosecution for CNCT19; the risk related to the Company's ongoing development of and regulatory application for CID-103 with respect to the treatment of antibody-mediated rejection for organ transplant and the license arrangements of CID-103; risks relating to interests of our largest shareholder and our Chairman and CEO that differ from our other shareholders; risks related to the development of a new manufacturing facility by CASI Pharmaceuticals (Wuxi) Co., Ltd. and risks related to our disagreement with Acrotech with respect to the termination of agreements regarding EVOMELA®. Further information regarding these and other risks is included in the Company's filings with the SEC. All information provided herein is as of the date of this announcement, and the Company undertakes no obligation to update any forward-looking statement, except as required under applicable law. We caution readers not to place undue reliance on any forward-looking statements contained herein.
EVOMELA® is proprietary to Acrotech Biopharma Inc. and its affiliates.FOLOTYN®is proprietary to Acrotech Biopharma Inc and its affiliates. The Company is currently involved in disputes and legal proceedings related to certain pipeline products, including EVOMELA® and CNCT-19.Please refer to the Company's filings with the U.S. Securities and Exchange Commission for further information.
COMPANY CONTACT:
Rui Zhang
CASI Pharmaceuticals, Inc.
240.864.2643
[email protected]
SOURCE: CASI Pharmaceuticals
View the original press release on ACCESS Newswire
L.Miller--AMWN