
-
 Chelsea's Mudryk charged over anti-doping violation
Chelsea's Mudryk charged over anti-doping violation
-
Draper survives scare to reach Queen's quarter-finals

-
 Pant hopes India can make country 'happy again' after plane crash
Pant hopes India can make country 'happy again' after plane crash
-
US Supreme Court upholds ban on gender-affirming care for minors

-
 UK risks more extreme, prolonged heatwaves in future: study
UK risks more extreme, prolonged heatwaves in future: study
-
Gosdens celebrate Royal Ascot double as Buick motors home on Ombudsman

-
 Oil prices drop following Trump's Iran comments, US stocks rise
Oil prices drop following Trump's Iran comments, US stocks rise
-
Musk's X sues to block New York social media transparency law

-
 Iran-Israel war: a lifeline for Netanyahu?
Iran-Israel war: a lifeline for Netanyahu?
-
Gaza Humanitarian Foundation initiative 'outrageous': UN probe chief

-
 India's Pant glad of Anderson and Broad exits ahead of England Tests
India's Pant glad of Anderson and Broad exits ahead of England Tests
-
Moth uses stars to navigate long distances, scientists discover

-
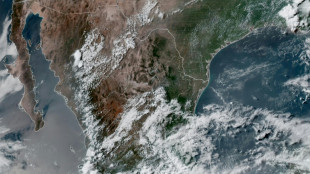 Hurricane Erick approaches Mexico's Pacific coast
Hurricane Erick approaches Mexico's Pacific coast
-
Gaza flotilla skipper vows to return

-
 Netherlands returns over 100 Benin Bronzes looted from Nigeria
Netherlands returns over 100 Benin Bronzes looted from Nigeria
-
Nippon, US Steel say they have completed partnership deal

-
 Almeida takes fourth stage of Tour of Switzerland with injured Thomas out
Almeida takes fourth stage of Tour of Switzerland with injured Thomas out
-
World champion Olga Carmona signs for PSG women's team

-
 Putin T-shirts, robots and the Taliban -- but few Westerners at Russia's Davos
Putin T-shirts, robots and the Taliban -- but few Westerners at Russia's Davos
-
Trump on Iran strikes: 'I may do it, I may not do it'

-
 Khamenei vows Iran will never surrender
Khamenei vows Iran will never surrender
-
Bangladesh tighten grip on first Sri Lanka Test

-
 England's Pope keeps place for India series opener
England's Pope keeps place for India series opener
-
Itoje to lead Lions for first time against Argentina

-
 Oil rises, stocks mixed as investors watch rates, conflict
Oil rises, stocks mixed as investors watch rates, conflict
-
Iran-Israel war: latest developments

-
 Iran threatens response if US crosses 'red line': ambassador
Iran threatens response if US crosses 'red line': ambassador
-
Iranians buying supplies in Iraq tell of fear, shortages back home

-
 UK's Catherine, Princess of Wales, pulls out of Royal Ascot race meeting
UK's Catherine, Princess of Wales, pulls out of Royal Ascot race meeting
-
Rape trial of France's feminist icon Pelicot retold on Vienna stage

-
 Khamenei says Iran will 'never surrender', warns off US
Khamenei says Iran will 'never surrender', warns off US
-
Oil prices dip, stocks mixed tracking Mideast unrest

-
 How Paris's Seine river keeps the Louvre cool in summer
How Paris's Seine river keeps the Louvre cool in summer
-
Welshman Thomas out of Tour of Switzerland as 'precautionary measure'

-
 UN says two Iran nuclear sites destroyed in Israel strikes
UN says two Iran nuclear sites destroyed in Israel strikes
-
South Africans welcome home Test champions the Proteas

-
 Middle Age rents live on in German social housing legacy
Middle Age rents live on in German social housing legacy
-
Israel targets nuclear site as Iran claims hypersonic missile attack

-
 China's AliExpress risks fine for breaching EU illegal product rules
China's AliExpress risks fine for breaching EU illegal product rules
-
Liverpool face Bournemouth in Premier League opener, Man Utd host Arsenal

-
 Heatstroke alerts issued in Japan as temperatures surge
Heatstroke alerts issued in Japan as temperatures surge
-
Liverpool to kick off Premier League title defence against Bournemouth

-
 Meta offered $100 mn bonuses to poach OpenAI employees: CEO Altman
Meta offered $100 mn bonuses to poach OpenAI employees: CEO Altman
-
Spain pushes back against mooted 5% NATO spending goal

-
 UK inflation dips less than expected in May
UK inflation dips less than expected in May
-
Oil edges down, stocks mixed but Mideast war fears elevated

-
 Energy transition: how coal mines could go solar
Energy transition: how coal mines could go solar
-
Australian mushroom murder suspect not on trial for lying: defence

-
 New Zealand approves medicinal use of 'magic mushrooms'
New Zealand approves medicinal use of 'magic mushrooms'
-
Suspects in Bali murder all Australian, face death penalty: police


MIRA Pharmaceuticals' Lead Drug Candidate Ketamir-2 First Manuscript Accepted for Publication in the Peer-Reviewed Journal Frontiers in Pharmacology
MIAMI, FL / ACCESS Newswire / June 18, 2025 / MIRA Pharmaceuticals, Inc (Nasdaq:MIRA) ("MIRA" or the "Company"), a clinical-stage pharmaceutical company developing novel therapeutics for neurologic, neuropsychiatric, and metabolic disorders, today announced that the first manuscript describing its lead drug candidate Ketamir-2, currently being evaluated in an ongoing Phase 1 clinical trial for neuropathic pain, has been accepted for publication in the peer-reviewed journal Frontiers in Pharmacology.
The article, titled "KETAMIR-2, A NEW MOLECULAR ENTITY AND NOVEL KETAMINE ANALOG," authored by Itzchak Angel, Ph.D., MIRA's Chief Scientific Advisor, highlights Ketamir-2's pharmacological differentiation from ketamine and its potential as a next-generation CNS therapeutic.
Peer Review Validates Differentiated Pharmacology and Safety
Acceptance into Frontiers in Pharmacology provides external scientific validation by independent experts, underscoring the rigor and credibility of MIRA's research. The publication confirms that Ketamir-2 was specifically engineered to overcome limitations associated with ketamine-such as poor oral bioavailability, dissociative side effects, and non-specific receptor binding.
Key Highlights from the Publication:
Highly Selective, Cleaner Mechanism: Ketamir-2 is a low-affinity NMDA receptor antagonist that selectively targets the NMDA PCP site. Unlike ketamine, Ketamir-2 showed no significant interaction with over 40 other receptors, transporters, or ion channel targets-including dopamine, opioid, serotonin, and monoaminergic systems-highlighting its clean pharmacological profile and reduced off-target effects.
No Hyperlocomotion, Even at High Doses: In contrast to ketamine, Ketamir-2 did not induce hyperlocomotion in preclinical models-a behavior associated with agitation and schizophrenia-like symptoms-suggesting a favorable neurobehavioral safety profile.
Demonstrated Antidepressant and Anxiolytic Activity: In validated behavioral models (Open Field Test, Elevated Plus Maze, Forced Swim Test), Ketamir-2 demonstrated clear anxiolytic and antidepressant-like effects. Ketamine, used as a control, either showed no benefit or limited effect in most tests.
Oral Delivery with Efficient Brain Penetration: All studies were conducted via the oral route. Ketamir-2 was shown to cross the blood-brain barrier and is not a substrate for P-glycoprotein, which often limits oral drug delivery to the brain. This may explain Ketamir-2's ability to maintain CNS activity despite its lower NMDA receptor affinity.
"We are honored to see our foundational research on Ketamir-2 published in a high-impact scientific journal," said Erez Aminov, CEO of MIRA. "This milestone adds meaningful scientific credibility and supports our confidence in Ketamir-2's differentiated mechanism, favorable safety profile, and broad clinical potential."
"This peer-reviewed publication provides clear validation of the differentiated pharmacological profile of Ketamir-2," added Dr. Itzchak Angel, Chief Scientific Advisor. "Its clean pharmacological profile and safety make it a compelling next-generation alternative to ketamine."
Clinical and Corporate Updates
MIRA also announced that its Phase 1 trial of Ketamir-2 is progressing as planned, with no safety concerns reported to date and dose escalation advancing. The Company expects to initiate a Phase 2a clinical trial in neuropathic pain by year-end 2025, pending regulatory clearance.
In addition, the Company is preparing new scientific data submissions and presentations to further support Ketamir-2's clinical development and potential across CNS-related conditions.
MIRA also reaffirmed that the acquisition of SKNY Pharmaceuticals, which includes a first-in-class oral CB1/CB2 inverse agonist for obesity and smoking cessation (SKNY-1), is progressing on track. The Company has submitted the required regulatory filings for the merger to the U.S. Securities and Exchange Commission (SEC).
The publication will be available upon release at: www.frontiersin.org/journals/pharmacology
Cautionary Note Regarding Forward-Looking Statements
This press release and the statements of MIRA's management related thereto contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will," and variations of these words or similar expressions that are intended to identify forward-looking statements. Any statements in this press release that are not historical facts may be deemed forward-looking. Any forward-looking statements in this press release are based on MIRA's current expectations, estimates, and projections only as of the date of this release and are subject to a number of risks and uncertainties (many of which are beyond MIRA's control) that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including related to MIRA's potential merger with SKNY Pharmaceuticals, Inc. These and other risks concerning MIRA's programs and operations are described in additional detail in the Annual Report on Form 10-K for the year ended December 31, 2024, and other SEC filings, which are on file with the SEC at www.sec.gov and MIRA's website at https://www.mirapharmaceuticals.com/investors/sec-filings. MIRA explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Helga Moya
[email protected]
(786) 432-9792
SOURCE: MIRA Pharmaceuticals
View the original press release on ACCESS Newswire
F.Bennett--AMWN