
-
 Draper survives scare to reach Queen's quarter-finals
Draper survives scare to reach Queen's quarter-finals
-
Pant hopes India can make country 'happy again' after plane crash

-
 US Supreme Court upholds ban on gender-affirming care for minors
US Supreme Court upholds ban on gender-affirming care for minors
-
UK risks more extreme, prolonged heatwaves in future: study

-
 Gosdens celebrate Royal Ascot double as Buick motors home on Ombudsman
Gosdens celebrate Royal Ascot double as Buick motors home on Ombudsman
-
Oil prices drop following Trump's Iran comments, US stocks rise

-
 Musk's X sues to block New York social media transparency law
Musk's X sues to block New York social media transparency law
-
Iran-Israel war: a lifeline for Netanyahu?

-
 Gaza Humanitarian Foundation initiative 'outrageous': UN probe chief
Gaza Humanitarian Foundation initiative 'outrageous': UN probe chief
-
India's Pant glad of Anderson and Broad exits ahead of England Tests

-
 Moth uses stars to navigate long distances, scientists discover
Moth uses stars to navigate long distances, scientists discover
-
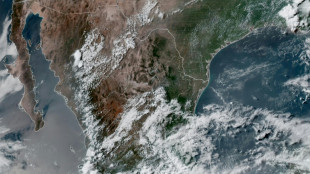
Hurricane Erick approaches Mexico's Pacific coast
-
 Gaza flotilla skipper vows to return
Gaza flotilla skipper vows to return
-
Netherlands returns over 100 Benin Bronzes looted from Nigeria

-
 Nippon, US Steel say they have completed partnership deal
Nippon, US Steel say they have completed partnership deal
-
Almeida takes fourth stage of Tour of Switzerland with injured Thomas out

-
 World champion Olga Carmona signs for PSG women's team
World champion Olga Carmona signs for PSG women's team
-
Putin T-shirts, robots and the Taliban -- but few Westerners at Russia's Davos

-
 Trump on Iran strikes: 'I may do it, I may not do it'
Trump on Iran strikes: 'I may do it, I may not do it'
-
Khamenei vows Iran will never surrender

-
 Bangladesh tighten grip on first Sri Lanka Test
Bangladesh tighten grip on first Sri Lanka Test
-
England's Pope keeps place for India series opener

-
 Itoje to lead Lions for first time against Argentina
Itoje to lead Lions for first time against Argentina
-
Oil rises, stocks mixed as investors watch rates, conflict

-
 Iran-Israel war: latest developments
Iran-Israel war: latest developments
-
Iran threatens response if US crosses 'red line': ambassador

-
 Iranians buying supplies in Iraq tell of fear, shortages back home
Iranians buying supplies in Iraq tell of fear, shortages back home
-
UK's Catherine, Princess of Wales, pulls out of Royal Ascot race meeting

-
 Rape trial of France's feminist icon Pelicot retold on Vienna stage
Rape trial of France's feminist icon Pelicot retold on Vienna stage
-
Khamenei says Iran will 'never surrender', warns off US

-
 Oil prices dip, stocks mixed tracking Mideast unrest
Oil prices dip, stocks mixed tracking Mideast unrest
-
How Paris's Seine river keeps the Louvre cool in summer

-
 Welshman Thomas out of Tour of Switzerland as 'precautionary measure'
Welshman Thomas out of Tour of Switzerland as 'precautionary measure'
-
UN says two Iran nuclear sites destroyed in Israel strikes

-
 South Africans welcome home Test champions the Proteas
South Africans welcome home Test champions the Proteas
-
Middle Age rents live on in German social housing legacy

-
 Israel targets nuclear site as Iran claims hypersonic missile attack
Israel targets nuclear site as Iran claims hypersonic missile attack
-
China's AliExpress risks fine for breaching EU illegal product rules

-
 Liverpool face Bournemouth in Premier League opener, Man Utd host Arsenal
Liverpool face Bournemouth in Premier League opener, Man Utd host Arsenal
-
Heatstroke alerts issued in Japan as temperatures surge

-
 Liverpool to kick off Premier League title defence against Bournemouth
Liverpool to kick off Premier League title defence against Bournemouth
-
Meta offered $100 mn bonuses to poach OpenAI employees: CEO Altman

-
 Spain pushes back against mooted 5% NATO spending goal
Spain pushes back against mooted 5% NATO spending goal
-
UK inflation dips less than expected in May

-
 Oil edges down, stocks mixed but Mideast war fears elevated
Oil edges down, stocks mixed but Mideast war fears elevated
-
Energy transition: how coal mines could go solar

-
 Australian mushroom murder suspect not on trial for lying: defence
Australian mushroom murder suspect not on trial for lying: defence
-
New Zealand approves medicinal use of 'magic mushrooms'

-
 Suspects in Bali murder all Australian, face death penalty: police
Suspects in Bali murder all Australian, face death penalty: police
-
Taiwan's entrepreneurs in China feel heat from cross-Strait tensions


Telomir Pharmaceuticals Prevents Cellular Aging in Patient-Derived Cells from Children with Progeria - an Ultra-Rare Genetic Disorder that Causes Rapid Aging
Study used cell lines obtained from the Progeria Research Foundation to evaluate Telomir-1's effects on key drivers of accelerated aging
MIAMI, FLORIDA / ACCESS Newswire / June 18, 2025 / Telomir Pharmaceuticals, Inc. (NASDAQ:TELO), a preclinical-stage biotechnology company focused on reversing biological aging and age-related diseases, today announced compelling new preclinical data showing that its lead candidate, Telomir-1, prevented cellular aging in human progeria cell lines obtained from the Progeria Research Foundation. Progeria, or Hutchinson-Gilford Progeria Syndrome (HGPS), is an ultra-rare pediatric disorder caused by a mutation in the LMNA gene. This mutation results in the production of an abnormal protein called progerin, which drives rapid biological aging in children.
There are an estimated 400-500 known cases worldwide, including fewer than 30 children currently living with the disease in the United States. Symptoms typically begin within the first two years of life and include growth failure, joint stiffness, loss of body fat and hair, and severe cardiovascular disease. Children with progeria have an average life expectancy of just 13 to 15 years, with most dying from heart attacks or strokes at a young age.
The only FDA-approved therapy for progeria, Zokinvy® (lonafarnib), is a farnesyltransferase inhibitor that has been shown to extend lifespan by an average of 4.3 years. However, Zokinvy does not reverse the underlying disease pathology or halt cardiovascular deterioration, which remains the leading cause of death. No approved therapy restores normal cell function or reverses the biological hallmarks of accelerated aging in progeria, highlighting a significant and urgent unmet medical need.
Telomir-1 is designed to regulate intracellular metal ions, reduce oxidative stress, restore mitochondrial function, extend telomere length, reverse muscle loss, and reset age-associated DNA methylation patterns - all of which are critical biological pathways implicated in progeria and broader age-related diseases.
In this study, conducted by Smart Assays, Telomir-1 was tested in cells taken directly from a child with HGPS. These cells were obtained from The Progeria Research Foundation (www.progeriaresearch.org). The study evaluated cell viability, reactive oxygen species (ROS), and intracellular calcium signaling - a marker of mitochondrial dysfunction - under both normal and stress-induced conditions.
Key findings include:
Improved cell viability: Telomir-1 increased survival in a dose-dependent manner, both under basal conditions and even under stress conditions induced by copper and iron-two metal ions known to accelerate aging by generating oxidative damage and destabilizing DNA and telomeres.
Reduction of oxidative stress: Progeria cells exhibited abnormally high levels of reactive oxygen species (ROS), a hallmark of cellular aging. Telomir-1 normalized these levels, both under basal conditions and even when ROS was further elevated by toxic metal exposure.
Restoration of mitochondrial function: Iron-induced calcium overload - a signal of mitochondrial damage and a known feature of HGPS - was significantly reduced with Telomir-1, indicating restored mitochondrial regulation and improved cellular energy balance.
These results demonstrate that Telomir-1 directly addresses the core cellular dysfunctions driving disease features in progeria - not only protecting cells from damage but restoring critical biological functions. The fact that these results were observed in actual patient-derived human cells offers strong early validation of Telomir-1's therapeutic potential.
"These results provide the strongest evidence to date that Telomir-1 is not only protective but also restorative at the molecular and cellular level," said Dr. Angel, Chief Scientific Advisor of Telomir. "What's especially promising is that the improvements we observed directly target the mechanisms known to drive disease progression in progeria - oxidative stress, metal toxicity, and mitochondrial instability. This level of functional rescue in actual patient-derived cells is highly encouraging as we move toward clinical translation. These studies come as further validation of the very promising results obtained previously in both nematode and zebrafish models of adult progeria."
"These findings deepen our conviction that Telomir-1 can be a first-in-class therapeutic platform for rare aging syndromes and broader age-related diseases," said Erez Aminov, CEO of Telomir. "By demonstrating the ability to reverse cellular damage in human progeria cells, Telomir-1 represents a potential breakthrough for children who currently have no real options beyond modestly delaying the inevitable. This work also lays the foundation for broader applications in neurodegeneration, metabolic dysfunction, and systemic aging.
The new data also build on previously reported studies in zebrafish and C. elegans nematodes harboring the wrn gene mutation (a model of adult progeria, or Werner syndrome), where Telomir-1 significantly extended lifespan, restored telomere length, reversed muscle degeneration, and normalized molecular age markers.
Telomir is currently finalizing IND-enabling studies for Telomir-1 and plans to engage with the U.S. Food and Drug Administration (FDA) to explore regulatory pathways, including the potential for orphan drug designation. The company is evaluating multiple rare disease indications for initial clinical development.
Cautionary Note Regarding Forward-Looking Statements
This press release, statements of Telomir's management or advisors related thereto, and the statements contained in the news story linked in this release contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These risks and uncertainties include, but are not limited to, the potential use of the data from our studies, our ability to develop and commercialize Telomir-1 for specific indications, and the safety of Telomir-1.
Any forward-looking statements in this press release are based on Telomir's current expectations, estimates and projections only as of the date of this release. These and other risks concerning Telomir's programs and operations are described in additional detail in its Annual Report on Form 10-K for the fiscal year ended December 31, 2024, which are on file with the SEC and available at www.sec.gov. Telomir explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact Information
Helga Moya
[email protected]
(786) 396-6723
SOURCE: Telomir Pharmaceuticals, Inc
View the original press release on ACCESS Newswire
A.Malone--AMWN