
-
 Oasis star Noel Gallagher piles praise on 'amazing' brother Liam
Oasis star Noel Gallagher piles praise on 'amazing' brother Liam
-
German minister says China's 'assertiveness' threatens European interests

-
 Markets waver as Japan exports show tariff strain
Markets waver as Japan exports show tariff strain
-
Afghanistan bus crash death toll rises to 78

-
 Historic Swedish church inches closer to new home
Historic Swedish church inches closer to new home
-
Asian markets waver as Japan exports show tariff strain

-
 Israel defence minister approves plan to conquer Gaza City
Israel defence minister approves plan to conquer Gaza City
-
More than 20 dead in fresh Pakistan monsoon rains

-
 Brazilian goalkeeper Fabio claims world record for most games
Brazilian goalkeeper Fabio claims world record for most games
-
Vienna chosen to host Eurovision 2026

-
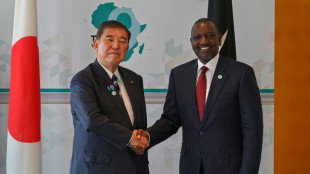 Japan hosts African leaders for development conference
Japan hosts African leaders for development conference
-
Reclusive Turkmenistan bids to go tobacco-free in 2025

-
 From TikTok to frontrunner, inside Paz's presidential campaign in Bolivia
From TikTok to frontrunner, inside Paz's presidential campaign in Bolivia
-
Chinese mega-hit 'Ne Zha II' enlists Michelle Yeoh to woo US audiences

-
 India celebrates clean energy milestone but coal still king
India celebrates clean energy milestone but coal still king
-
US demand for RVs fuels deforestation on Indonesia's Borneo: NGOs

-
 Kneecap rapper faces court on terror charge over Hezbollah flag
Kneecap rapper faces court on terror charge over Hezbollah flag
-
Dutch divers still haul up debris six years after container spill

-
 Asian markets dip after US tech slide
Asian markets dip after US tech slide
-
NZ soldier sentenced to two years' detention for attempted espionage

-
 Time to Go: Japan pro board game player retires at 98
Time to Go: Japan pro board game player retires at 98
-
City girls snub traditional Hindu face tattoos in Pakistan

-
 Australia lashes Netanyahu over 'weak' leader outburst
Australia lashes Netanyahu over 'weak' leader outburst
-
Polar bear waltz: Fake Trump-Putin AI images shroud Ukraine peace effort

-
 Sounds serious: NYC noise pollution takes a toll
Sounds serious: NYC noise pollution takes a toll
-
Trump slams US museums for focus on 'how bad slavery was'

-
 US agrees to talks with Brazilian WTO delegates on tariffs
US agrees to talks with Brazilian WTO delegates on tariffs
-
Israel-France row flares over Macron's move to recognise Palestinian state

-
 DEA Unconstitutional Marijuana Playbook: How the DEA Is Repeating the DOL's Mistakes Against MMJ BioPharma
DEA Unconstitutional Marijuana Playbook: How the DEA Is Repeating the DOL's Mistakes Against MMJ BioPharma
-
Nano One Successfully Commissions Proprietary Agitator Equipment Boosting Throughput Capacity at Candiac Five New Patents Added to Global IP Portfolio

-
 BioNxt Fast-Tracks U.S. Patent for MS Drug and Secures Broad Platform IP Covering Autoimmune Neurology Pipeline
BioNxt Fast-Tracks U.S. Patent for MS Drug and Secures Broad Platform IP Covering Autoimmune Neurology Pipeline
-
Tocvan Strengthens Team Appoints Christopher Gordon As Head Of Corporate Development

-
 ANGLE Announces Collaboration with Myriad Genetics
ANGLE Announces Collaboration with Myriad Genetics
-
White House starts TikTok account as platform in US legal limbo

-
 Syrian, Israeli diplomats met in Paris to discuss 'de-escalation': report
Syrian, Israeli diplomats met in Paris to discuss 'de-escalation': report
-
Wanyonyi, the former cattle herder ready to eclipse Rudisha

-
 Swiatek, Ruud romp into US Open mixed doubles semis, Alcaraz, Djokovic out
Swiatek, Ruud romp into US Open mixed doubles semis, Alcaraz, Djokovic out
-
Mbappe lifts Real Madrid past Osasuna in La Liga opener

-
 Venezuela says 66 children 'kidnapped' by the United States
Venezuela says 66 children 'kidnapped' by the United States
-
Brazil nixes red World Cup jersey amid political outcry

-
 Real Madrid scrape past Osasuna in La Liga opener
Real Madrid scrape past Osasuna in La Liga opener
-
McIlroy backs 'clean slate' season finale format change

-
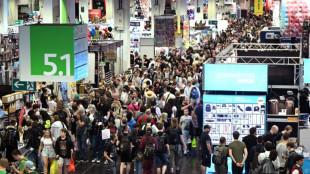 'Call of Duty', 'Black Myth' wow Gamescom trade show
'Call of Duty', 'Black Myth' wow Gamescom trade show
-
Isak says 'change' best for everyone after Newcastle trust broken

-
 Salah makes history with third PFA player of the year award
Salah makes history with third PFA player of the year award
-
Rabiot, Rowe put up for sale by Marseille after bust-up

-
 Weary Swiatek wins US Open mixed doubles opener
Weary Swiatek wins US Open mixed doubles opener
-
Miami fearing Messi blow ahead of Leagues Cup quarter-finals

-
 Trump rules out US troops but eyes air power in Ukraine deal
Trump rules out US troops but eyes air power in Ukraine deal
-
Trump course back on PGA schedule for 2026 season: tour


MIRA Reports Potent Inflammatory Pain Relief from Non-Psychoactive Marijuana Analog Mira-55 in Animal Model, Matching Morphine Without Opioid Risks
With Mira-55 and Ketamir-2, MIRA is advancing complementary non-opioid therapies for two of the largest pain markets
MIAMI, FLORIDA / ACCESS Newswire / July 3, 2025 / MIRA Pharmaceuticals, Inc. (NASDAQ:MIRA) ("MIRA" or the "Company"), a clinical-stage pharmaceutical company developing novel therapeutics for neurologic, neuropsychiatric, and metabolic disorders, today announced positive preclinical data demonstrating that Mira-55, the Company's proprietary non-psychotropic marijuana analog, delivered morphine-comparable pain relief in a validated model of inflammatory pain-without causing local inflammation.
Mira-55 is a next-generation analog of marijuana, engineered to selectively activate CB2 cannabinoid receptors, which are associated with anti-inflammatory and analgesic effects. Unlike THC, Mira-55 minimizes activation of CB1 receptors, reducing the risk of euphoria, sedation, and pro-inflammatory side effects.
"These results reinforce the value of Mira-55 as a differentiated cannabinoid-based therapy with real clinical potential," said Erez Aminov, Chairman and CEO of MIRA. "We believe the drug's ability to match morphine's pain relief-without the baggage of addiction, sedation, or THC-like effects-makes Mira-55 an ideal candidate for large, underserved inflammatory pain markets. It's another step in building a non-opioid pain franchise that addresses both inflammatory and neuropathic pain."
Study Overview and Key Findings
Mira-55 was tested using the formalin model, a gold-standard preclinical method for studying inflammatory pain. In this model, formalin is injected into the rat's paw, producing a pain response that mimics human inflammatory pain. Pain sensitivity was assessed using Von Frey Filament testing, which measures tactile pain thresholds, and inflammation was measured by paw edema volume.
Key findings:
Mira-55 reduced pain sensitivity by approximately threefold, restoring thresholds to near-baseline levels.
Its analgesic effect was equivalent to morphine, the standard opioid comparator in the study.
No sedation or inflammatory swelling was observed with Mira-55 treatment.
Importantly, following a scientific review, the U.S. Drug Enforcement Administration (DEA) determined that Mira-55 is not classified as a controlled substance. This designation supports the compound's long-term clinical and commercial viability and removes key barriers typically associated with cannabinoid-based drug development.
These results build on prior data from a separate inflammatory pain model conducted by a leading U.S. academic research center, where Mira-55 blocked both thermal and mechanical hyperalgesia without increasing inflammation. In contrast, low-dose THC in that model exacerbated inflammation-further validating Mira-55's selective pharmacological profile.
"Mira-55 offers the pain-relieving potential of cannabinoids without the liabilities traditionally seen in THC-based drugs," said Dr. Itzchak Angel, Chief Scientific Advisor at MIRA. "Its novel structure and unique profile with CB2 selectivity and non-scheduled DEA status make it a compelling candidate for treating inflammation-driven pain conditions that are poorly managed by today's standards."
Strategic Fit Within MIRA's Pain Portfolio
Mira-55 complements Ketamir-2, MIRA's clinical-stage NMDA receptor antagonist, which is advancing through Phase 1 development for neuropathic pain. While Ketamir-2 addresses nerve-related pain through central mechanisms, Mira-55 targets inflammatory pain through the endocannabinoid system. Together, they represent two mechanistically distinct, non-opioid approaches to treating chronic pain.
"With Mira-55 and Ketamir-2, we now have two highly differentiated drug candidates with the potential to transform how inflammatory and neuropathic conditions are treated," added Aminov. "We're advancing each asset methodically, and we're energized by the momentum we've built across the pipeline."
Corporate Update on SKNY Merger
MIRA also announced continued progress on its previously disclosed acquisition of SKNY Pharmaceuticals, the developer of SKNY-1, a novel investigational therapy targeting both obesity and nicotine addiction. In recent studies, SKNY-1 demonstrated a 30% reduction in body weight without muscle loss, along with a reversal of nicotine cravings-highlighting its potential as a differentiated treatment in two major markets. The U.S. Securities and Exchange Commission (SEC) has completed its review of the merger proxy with no comments, allowing MIRA to proceed with shareholder approval and the final steps toward completing the transaction.
"We are pleased to report that the SEC had no comments on our merger filing, which reflects the quality of our regulatory and business preparation," said Aminov. "This milestone allows us to advance toward shareholder approval with clarity and confidence as we prepare for the next phase of growth."
Next Steps
MIRA Pharmaceuticals is advancing Mira-55 toward an Investigational New Drug (IND) submission, with ongoing activities supporting future clinical development in inflammatory pain. The Company remains focused on progressing both lead programs-Mira-55 and Ketamir-2-toward their next regulatory and clinical milestones.
About MIRA Pharmaceuticals, Inc.
MIRA Pharmaceuticals, Inc. (NASDAQ:MIRA) is a clinical-stage pharmaceutical company focused on the development and commercialization of novel therapeutics for neurologic, neuropsychiatric, and metabolic disorders. The Company's pipeline includes oral drug candidates designed to address significant unmet medical needs in areas such as neuropathic pain, inflammatory pain, obesity, addiction, anxiety, and cognitive decline.
Cautionary Note Regarding Forward-Looking Statements
This press release and the statements of MIRA's management related thereto contain "forward-looking statements," which are statements other than historical facts made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will," and variations of these words or similar expressions that are intended to identify forward-looking statements. Any statements in this press release that are not historical facts may be deemed forward-looking. Any forward-looking statements in this press release are based on MIRA's current expectations, estimates, and projections only as of the date of this release and are subject to a number of risks and uncertainties (many of which are beyond MIRA's control) that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including related to MIRA's potential merger with SKNY Pharmaceuticals, Inc. These and other risks concerning MIRA's programs and operations are described in additional detail in the Annual Report on Form 10-K for the year ended December 31, 2024, and the Form 14A filed by MIRA on June 18, 2025, and other SEC filings, which are on file with the SEC at www.sec.gov and on MIRA's website at https://www.mirapharmaceuticals.com/investors/sec-filings. MIRA explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Contact:
Helga Moya
[email protected]
(786) 432-9792
SOURCE: MIRA Pharmaceuticals
View the original press release on ACCESS Newswire
P.Stevenson--AMWN