
-
 Snapchat begins age checks in Australia ahead of social media ban
Snapchat begins age checks in Australia ahead of social media ban
-
Ukrainian sumo wrestler shocked to win first title

-
 Shai scores 37 as NBA-best Thunder avenge lone loss
Shai scores 37 as NBA-best Thunder avenge lone loss
-
McCullum tells England fans to 'keep the faith' after Perth capitulation

-
 UK far-right figures stoke digital ID fears with unproven claims
UK far-right figures stoke digital ID fears with unproven claims
-
Messi magic as Miami thrash Cincinnati, face NYCFC next

-
 Asian stocks rise as US rate hopes soothe nerves after torrid week
Asian stocks rise as US rate hopes soothe nerves after torrid week
-
Rising from the ashes: Mogadishu's building boom

-
 Ronaldo, 40, scores spectacular bicycle kick
Ronaldo, 40, scores spectacular bicycle kick
-
New Zealand's Williamson back for West Indies Tests

-
 Washington, Kyiv say a peace deal must 'fully uphold' Ukraine sovereignty
Washington, Kyiv say a peace deal must 'fully uphold' Ukraine sovereignty
-
EU, Africa leaders to talk trade and minerals, as Ukraine looms large

-
 Rhino horn sales, shark protection on wildlife talks agenda
Rhino horn sales, shark protection on wildlife talks agenda
-
Mexico training ship that hit Brooklyn Bridge returns home

-
 Messi magic as 'perfect' Miami thrash Cincinnati
Messi magic as 'perfect' Miami thrash Cincinnati
-
Despite doubts over economy, Americans set for record holiday spending

-
 Messi magic sends Miami into MLS conference final
Messi magic sends Miami into MLS conference final
-
Australian mining giant BHP drops Anglo American takeover bid

-
 Real Madrid drop points at Elche, Atletico scrape win
Real Madrid drop points at Elche, Atletico scrape win
-
Bellingham rescues Real Madrid draw at Elche

-
 Mahomes rescue act saves Chiefs as Colts stumble
Mahomes rescue act saves Chiefs as Colts stumble
-
Finland's Valimaki wins first PGA title at RSM Classic

-
 Pulisic fires AC Milan to derby glory as Roma move top of Serie A
Pulisic fires AC Milan to derby glory as Roma move top of Serie A
-
50 children in Nigeria escape after kidnapping as 38 worshippers rescued

-
 Giroud brace earns Lille win over Paris FC in Ligue 1
Giroud brace earns Lille win over Paris FC in Ligue 1
-
US says 'very optimistic' on Ukraine plan as Geneva talks end

-
 No.1 Jeeno defends title at LPGA Tour Championship
No.1 Jeeno defends title at LPGA Tour Championship
-
Slovenia referendum rejects contested assisted dying law

-
 Bolsonaro says ankle monitor tampering driven by 'paranoia'
Bolsonaro says ankle monitor tampering driven by 'paranoia'
-
Argentina boss Contepomi labels Curry a 'bully' after England loss

-
 Sinner-less Italy see off Spain to complete Davis Cup hat-trick
Sinner-less Italy see off Spain to complete Davis Cup hat-trick
-
'Exceptional' Eze deserved spotlight in Arsenal rout of Spurs: Arteta

-
 Atletico snatch late win at Getafe with own-goal
Atletico snatch late win at Getafe with own-goal
-
US, Ukraine envoys hail 'good progress' at talks to end Russia war

-
 Italy see off Spain to complete Davis Cup hat-trick
Italy see off Spain to complete Davis Cup hat-trick
-
Arsenal's Eze revels in 'special' hat-trick after destroying Spurs

-
 Ouedraogo stunner sends Leipzig second in Bundesliga
Ouedraogo stunner sends Leipzig second in Bundesliga
-
Ojomoh stars as England edge Argentina for 11th win in a row

-
 Eze treble fuels Arsenal's derby rout of Spurs
Eze treble fuels Arsenal's derby rout of Spurs
-
'Wicked' sequel sees green in weekend-winning N. America debut

-
 Ojomoh stars as England edge Argentina in Twickenham thriller
Ojomoh stars as England edge Argentina in Twickenham thriller
-
Israel says killed Hezbollah chief of staff in Beirut strike

-
 Roma take top spot in Serie A ahead of Milan derby
Roma take top spot in Serie A ahead of Milan derby
-
Berrettini puts Italy on verge of third straight Davis Cup triumph

-
 Trump blasts Ukraine for 'zero gratitude' amid talks to halt war
Trump blasts Ukraine for 'zero gratitude' amid talks to halt war
-
Ouedraogo stunner sends Leipzig second

-
 What does US 'terrorist' designation for Venezuela mean?
What does US 'terrorist' designation for Venezuela mean?
-
Israel targets Hezbollah chief of staff in deadly Beirut strike

-
 Scotland thrash Tonga in Autumn Nations finale
Scotland thrash Tonga in Autumn Nations finale
-
Three key Irish takeaways from Autumn Nations Series


Florence Connects 65,000+ Study Sites and 600+ Sponsors Worldwide - Unleashing the Next Era of Clinical Trial Intelligence
With the industry's largest network of sites, Florence sets a new benchmark for AI-enabled study startup, workflow automation, and operational risk management.
ATLANTA, GA / ACCESS Newswire / October 23, 2025 / Florence Healthcare reinforced its position as the global leader in clinical trial operations with a major milestone: Florence Trial Operations Platform now connects 65,000 study sites spanning more than 600 sponsors across 90+ countries, forming the industry's largest network. Recognized as the #1 clinical trial technology for six consecutive years, Florence continues to set the standard for startup speed, workflow automation, and operational risk management across sponsor study portfolios.
"Florence was built to bring sponsors and sites into a shared operational space," said Ryan Jones, CEO of Florence Healthcare. "First, we fully digitized startup to eliminate manual bottlenecks and paper workflows. Now, we're using AI to enhance speed and augment intelligence across 65,000 study sites globally - arming sponsors with portfolio-wide operational visibility so they can anticipate trial risk, act on insights, and drive execution on the ground."
Solving the $1 million per study problem
While digital-first sites set new standards for operational speed and efficiency, much of the industry still remains offline. Only 30% of global sites currently use an eISF, leaving roughly 200,000 sites dependent on inefficient paper processes for study startup, document collection and storage, regulatory process management, monitoring and closeout.
This lack of digitization costs sponsors an estimated $1 million per study in lost productivity, rework, quality and compliance risk.
Florence is closing this digital divide through the fastest growing clinical research network of 65,000 study sites and 600+ sponsors. By digitizing 100% of operational workflows, Florence sets the standard for startup speed, operational cost reduction, and risk control.
The results are compelling:
Up to 70% faster last mile study startup operations compared to the average site startup time for a Top 10 global pharmaceutical sponsor.
$141M annual operational cost takeout by automating site readiness workflows, based on active daily users and total hours saved per year across Florence's study site network.
51% increase in eTMF QA pass rates from 65% to 98.7%, due to built-in operational audits and automated compliance checks to control risk.
Shaping the Future of Study Operations
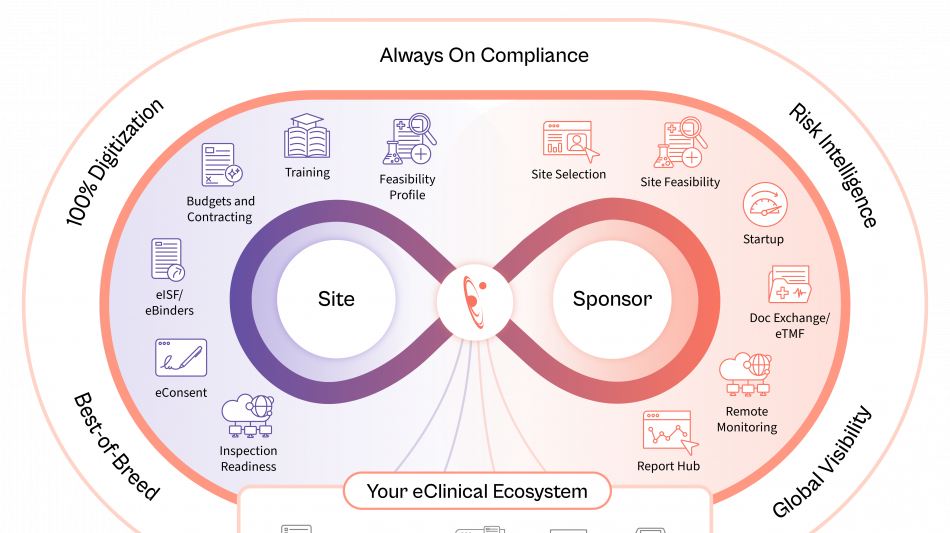
Florence is embedding AI across the study lifecycle to unlock operational data intelligence, recommend next-best actions, strengthen risk control, and improve operating efficiency.
Site Identification & Feasibility
Drawing on the industry's largest network of site intelligence, Florence enables sponsors and CROs to identify the most qualified sites by therapeutic area, geography, and performance, while AI-assisted feasibility surveys ensure faster, more accurate completion.
Study Startup
AI-enabled contracting and document exchange between eTMF and eISF automates SSU document exchange, while generative AI redlining shortens contract review cycles, compares terms in seconds, and dramatically reduces time to activation.
Remote Monitoring
AI-powered reporting surfaces early risk signals from operational audit trails and site data, providing real-time visibility and enabling proactive intervention. These insights minimize the need for on-site visits and ensure trials stay on schedule.
Together, these advancements move sponsors and CROs from manual oversight to intelligent execution across global study portfolios. Through its open API network, Florence rapidly integrates and extends its trial operation capabilities to other eClinical partner systems.
Florence AI operates at the speed of trust, ensuring every study remains fully aligned with FDA, EMA, HIPAA, GDPR, EU Annex 11, ICH E6 (R3), and GCP standards.
All capabilities will be available in December 2025.
Join the Movement: Research Revolution 2025
Florence will showcase its newest capabilities at Research Revolution 2025, the company's annual global event (October 26-28, 2025) bringing together sponsors, CROs, and research sites. Be part of the global revolution. Watch it live at: https://researchrevolutionsummit.com/live/
About Florence Healthcare
Florence is a purpose-built platform that connects sponsors and sites to accelerate clinical trials, improve operational capacity, and reduce risk. Designed for scale, Florence streamlines workflows, enhances collaboration, and delivers real-time visibility across studies-empowering research teams to move faster, stay inspection-ready, and increase trial throughput with fewer resources.
Contact Information
Seema Sheth-Voss
[email protected]
(888) 829-0896
SOURCE: Florence Healthcare
View the original press release on ACCESS Newswire
L.Durand--AMWN
