
-
 Scotland thrash Tonga in Autumn Nations finale
Scotland thrash Tonga in Autumn Nations finale
-
Three key Irish takeaways from Autumn Nations Series

-
 Imperious Shiffrin swoops to 103rd win at Gurgl
Imperious Shiffrin swoops to 103rd win at Gurgl
-
Schmidt challenges Wallabies to 'roll up their sleeves' after gruesome year

-
 Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva
Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva
-
South African centurion Muthusamy celebrates 'awesome' Test journey

-
 Brazil 'very concerned' about US naval build-up near Venezuela
Brazil 'very concerned' about US naval build-up near Venezuela
-
Liverpool a 'mess' says Van Dijk

-
 First blind women's T20 cricket World Cup boosts sport
First blind women's T20 cricket World Cup boosts sport
-
France eye Dupont boost for Six Nations defence

-
 McLaren boss apologises to Norris, Piastri for Vegas disqualification
McLaren boss apologises to Norris, Piastri for Vegas disqualification
-
G20 grapples with splintering world order

-
 Verstappen wins big in Vegas with McLarens disqualified
Verstappen wins big in Vegas with McLarens disqualified
-
Muthusamy, Jansen put South Africa on top in second India Test

-
 Rubio lands in Geneva for talks on Ukraine plan
Rubio lands in Geneva for talks on Ukraine plan
-
Norris and Piastri disqualified from Las Vegas GP

-
 Slovenia holds crunch vote on contested assisted dying law
Slovenia holds crunch vote on contested assisted dying law
-
Aonishiki beomes first Ukrainian to win sumo tournament

-
 Holders Australia drawn with New Zealand in Rugby League World Cup
Holders Australia drawn with New Zealand in Rugby League World Cup
-
Vietnam flooding kills at least 90

-
 Muthusamy's maiden Test century powers South Africa to 428-7
Muthusamy's maiden Test century powers South Africa to 428-7
-
Myanmar junta says nearly 1,600 foreigners arrested in scam hub raids

-
 US signals room for negotiation on Ukraine plan ahead of talks
US signals room for negotiation on Ukraine plan ahead of talks
-
Verstappen wins Las Vegas F1 Grand Prix, Norris edges closer to crown

-
 Muthusamy anchors South Africa to 316-6 in second India Test
Muthusamy anchors South Africa to 316-6 in second India Test
-
Vietnam flood death toll rises to 90

-
 US denies pushing Russian 'wish list' as Ukraine plan
US denies pushing Russian 'wish list' as Ukraine plan
-
Harden's 55 leads Clippers win as Pistons streak hits 12

-
 Kim's first top-10 in 14 years as Ballester wins maiden pro title
Kim's first top-10 in 14 years as Ballester wins maiden pro title
-
Gotham crowned NWSL champions after Lavelle breaks Spirit
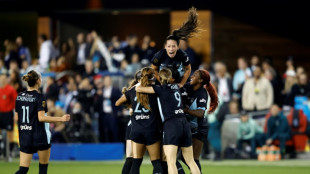
-
 Trump signals room for negotiation on Ukraine plan ahead of talks
Trump signals room for negotiation on Ukraine plan ahead of talks
-
Head shapes up as solution for Australia's opening woes

-
 Tomorrowland bets on Chinese dance music fans with first indoor event
Tomorrowland bets on Chinese dance music fans with first indoor event
-
England slammed as 'brainless' after first Ashes Test capitulation

-
 Slovenia to hold new vote on contested assisted dying law
Slovenia to hold new vote on contested assisted dying law
-
10 Benefits of Choosing Dental Implants After an Extraction

-
 SKYLINE Announces Q3 2025 Financial Results
SKYLINE Announces Q3 2025 Financial Results
-
'Beer tastes better' for Eramsus after win over Irish

-
 No.1 Jeeno leads by six at LPGA Tour Championship
No.1 Jeeno leads by six at LPGA Tour Championship
-
Neres double fires Napoli top in Italy

-
 Bielle-Biarrey masterclass helps France hold off Australia
Bielle-Biarrey masterclass helps France hold off Australia
-
Pogba returns in Monaco loss as PSG stay top in France

-
 COP30: Key reactions to climate deal
COP30: Key reactions to climate deal
-
What did countries agree to at COP30?

-
 Harden's club-record 55 points leads Clippers over Hornets
Harden's club-record 55 points leads Clippers over Hornets
-
Amazon climate deal a 'win' for global unity but fossil fuels untouched

-
 Boos, blowups and last-minute pause as a chaotic COP30 closes out
Boos, blowups and last-minute pause as a chaotic COP30 closes out
-
Farrell proud of Ireland after 'mad' Test with South Africa

-
 Gaza civil defence says 21 killed in Israeli strikes
Gaza civil defence says 21 killed in Israeli strikes
-
South Africa beat ill-disciplined Irish to end Dublin drought


Protagenic Therapeutics Announces Completion of Enrollment and Dosing in Phase 1 MAD Study
NEW YORK CITY, NY / ACCESS Newswire / November 13, 2025 / Protagenic Therapeutics Inc. (NASDAQ:PTIX), a biopharmaceutical company developing novel therapeutics for stress-related neuropsychiatric and neurodegenerative disorders, today announced the successful completion of enrollment and dosing in its Multiple Ascending Dose (MAD) Phase 1 study evaluating its lead compound, known as PT00114. The study, conducted in healthy volunteers, was designed to assess the safety and tolerability profile of PT00114 following multiple doses over time. With all healthy volunteers now dosed, the company has achieved a milestone in the clinical development of its proprietary peptide-based therapeutic.
"We've completed all dosing for all healthy volunteers in the Phase 1 Study," said Dr. Garo Armen, Executive Chairman of Protagenic Therapeutics. "We plan to deliver a full summation of our analysis of the safety results within by the end of the month."
The company expects to finalize and release top-line results from the MAD study by November 30th.
"This milestone underscores our commitment to advancing PT00114 as a novel, first-in-class therapeutic aimed at restoring resilience and balance in individuals suffering from chronic stress and its neurological consequences," added Dr. Armen. "The company had previously completed a single ascending dose study in early 2025. We are encouraged by the progress to date and are excited to move toward the next phase of development."
About Protagenic Therapeutics, Inc.
Protagenic Therapeutics, Inc. (NASDAQ:PTIX) is a biopharmaceutical company focused on developing innovative peptide-based therapeutics targeting the biological pathways underlying stress-related neurological and mood disorders. The company's lead compound, PT00114, is a synthetic analog of a naturally occurring brain peptide that helps regulate the body's stress response and emotional equilibrium. For more information, visit www.protagenic.com.
Forward-Looking Statements
Statements in this press release contain "forward-looking statements," within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "suggest," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on the Company's current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including risks related to the completion, timing and results of current and future clinical studies relating to the Company's product candidates. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled "Risk Factors" in the Company's Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission. Forward-looking statements contained in this announcement are made as of this date, and the Company undertakes no duty to update such information except as required under applicable law.
Company Contact:
Alexander K. Arrow, MD, CFA
Chief Financial Officer
Protagenic Therapeutics, Inc.
149 Fifth Ave, Suite 500, New York, NY 10010
Tel: 213-260-4342
Email: [email protected]
SOURCE: Protagenic Therapeutics, Inc.
View the original press release on ACCESS Newswire
A.Rodriguezv--AMWN


