
-
 Scotland thrash Tonga in Autumn Nations finale
Scotland thrash Tonga in Autumn Nations finale
-
Three key Irish takeaways from Autumn Nations Series

-
 Imperious Shiffrin swoops to 103rd win at Gurgl
Imperious Shiffrin swoops to 103rd win at Gurgl
-
Schmidt challenges Wallabies to 'roll up their sleeves' after gruesome year

-
 Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva
Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva
-
South African centurion Muthusamy celebrates 'awesome' Test journey

-
 Brazil 'very concerned' about US naval build-up near Venezuela
Brazil 'very concerned' about US naval build-up near Venezuela
-
Liverpool a 'mess' says Van Dijk

-
 First blind women's T20 cricket World Cup boosts sport
First blind women's T20 cricket World Cup boosts sport
-
France eye Dupont boost for Six Nations defence

-
 McLaren boss apologises to Norris, Piastri for Vegas disqualification
McLaren boss apologises to Norris, Piastri for Vegas disqualification
-
G20 grapples with splintering world order

-
 Verstappen wins big in Vegas with McLarens disqualified
Verstappen wins big in Vegas with McLarens disqualified
-
Muthusamy, Jansen put South Africa on top in second India Test

-
 Rubio lands in Geneva for talks on Ukraine plan
Rubio lands in Geneva for talks on Ukraine plan
-
Norris and Piastri disqualified from Las Vegas GP

-
 Slovenia holds crunch vote on contested assisted dying law
Slovenia holds crunch vote on contested assisted dying law
-
Aonishiki beomes first Ukrainian to win sumo tournament

-
 Holders Australia drawn with New Zealand in Rugby League World Cup
Holders Australia drawn with New Zealand in Rugby League World Cup
-
Vietnam flooding kills at least 90

-
 Muthusamy's maiden Test century powers South Africa to 428-7
Muthusamy's maiden Test century powers South Africa to 428-7
-
Myanmar junta says nearly 1,600 foreigners arrested in scam hub raids

-
 US signals room for negotiation on Ukraine plan ahead of talks
US signals room for negotiation on Ukraine plan ahead of talks
-
Verstappen wins Las Vegas F1 Grand Prix, Norris edges closer to crown

-
 Muthusamy anchors South Africa to 316-6 in second India Test
Muthusamy anchors South Africa to 316-6 in second India Test
-
Vietnam flood death toll rises to 90

-
 US denies pushing Russian 'wish list' as Ukraine plan
US denies pushing Russian 'wish list' as Ukraine plan
-
Harden's 55 leads Clippers win as Pistons streak hits 12

-
 Kim's first top-10 in 14 years as Ballester wins maiden pro title
Kim's first top-10 in 14 years as Ballester wins maiden pro title
-
Gotham crowned NWSL champions after Lavelle breaks Spirit
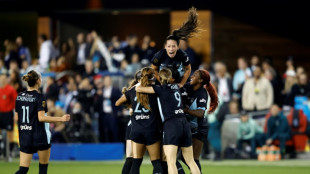
-
 Trump signals room for negotiation on Ukraine plan ahead of talks
Trump signals room for negotiation on Ukraine plan ahead of talks
-
Head shapes up as solution for Australia's opening woes

-
 Tomorrowland bets on Chinese dance music fans with first indoor event
Tomorrowland bets on Chinese dance music fans with first indoor event
-
England slammed as 'brainless' after first Ashes Test capitulation

-
 Slovenia to hold new vote on contested assisted dying law
Slovenia to hold new vote on contested assisted dying law
-
10 Benefits of Choosing Dental Implants After an Extraction

-
 SKYLINE Announces Q3 2025 Financial Results
SKYLINE Announces Q3 2025 Financial Results
-
'Beer tastes better' for Eramsus after win over Irish

-
 No.1 Jeeno leads by six at LPGA Tour Championship
No.1 Jeeno leads by six at LPGA Tour Championship
-
Neres double fires Napoli top in Italy

-
 Bielle-Biarrey masterclass helps France hold off Australia
Bielle-Biarrey masterclass helps France hold off Australia
-
Pogba returns in Monaco loss as PSG stay top in France

-
 COP30: Key reactions to climate deal
COP30: Key reactions to climate deal
-
What did countries agree to at COP30?

-
 Harden's club-record 55 points leads Clippers over Hornets
Harden's club-record 55 points leads Clippers over Hornets
-
Amazon climate deal a 'win' for global unity but fossil fuels untouched

-
 Boos, blowups and last-minute pause as a chaotic COP30 closes out
Boos, blowups and last-minute pause as a chaotic COP30 closes out
-
Farrell proud of Ireland after 'mad' Test with South Africa

-
 Gaza civil defence says 21 killed in Israeli strikes
Gaza civil defence says 21 killed in Israeli strikes
-
South Africa beat ill-disciplined Irish to end Dublin drought


Jaguar Health Provides Recap of November 8, 2025 Presentation on Groundbreaking Results of Proof-of-Concept Study of Crofelemer for Treatment of Pediatric Intestinal Failure at NASPGHAN Annual Meeting
Crofelemer can potentially extend and save lives of microvillus inclusion disease patients, reducing the volume of the total parenteral support (PS) necessary for them to survive
Groundbreaking PS reduction of up to 37% is unprecedented; No approved treatments exist for MVID
SAN FRANCISCO, CA / ACCESS Newswire / November 13, 2025 / Jaguar Health, Inc. (NASDAQ:JAGX) (Jaguar) family company Napo Pharmaceuticals (Napo) today provided a recap of the November 8, 2025 presentation at the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Annual Meeting describing the initial groundbreaking results of an independent investigator-initiated trial (IIT) of crofelemer in the UAE for treatment of pediatric intestinal failure, which includes patients with 'intestinal failure' due to microvillus inclusion disease (MVID) and short bowel syndrome (SBS-IF).
"The intestines of patients with the ultrarare genetic disorder MVID do not function properly, as is often the case with patients having intestinal failure due to short bowel syndrome. The intestines of these patients are unable to absorb the fluids, electrolytes and nutrients required to survive and thrive. Intestinal failure is a debilitating, lifelong condition that often requires patients to receive life-sustaining fluids, electrolytes and nutrients through intravenous administration, which consists of total parenteral nutrition (TPN) with supplemental intravenous fluids, which together constitute parenteral support (PS). Most intestinal failure patients require PS up to 7 days a week, and sometimes for 20 hours or more per day," said Lisa Conte, Jaguar's founder, president, and CEO.
While crucial for these patients, total parenteral support is associated with significant toxicities to patients, similar to some toxicities associated with chemotherapy, often causing serious health problems including infections, metabolic complications, liver and kidney function problems - as well as a risk of neurodevelopmental delay. These symptoms may emerge at any time in intestinal failure patients, and often become life-threatening.
As presented at NASPGHAN, the results of this ongoing and independent proof-of-concept trial of crofelemer demonstrate disease progression modification through reduction of total parenteral support (PS) in pediatric intestinal failure patients that ranged from 12 to 37%. Specifically, in the two SBS-IF patients who have completed treatment, the results show crofelemer reduced PS between 12.5 to 15.6% at the highest dose over the 12-week treatment period, together with reduced loose watery stools frequency. For the initial MVID patient who has completed treatment, PS needs were reduced by up to 27% at the highest dose over the initial 12-week treatment period and up to 37% during the extension period upon reinitiation of crofelemer treatment, and also showed reduced frequency of loose watery stools. These findings support continued evaluation of crofelemer to reduce PS needs for pediatric intestinal failure patients.
The presentation on November 8, 2025 at NASPGHAN, was titled Exploratory Single-Arm, Open-Label Non-Randomized Trial Evaluating the Safety and Effectiveness of Crofelemer in Pediatric Patients with Intestinal Failure. The study's primary investigator, Dr. Mohamad Miqdady, Division Chief of the Pediatric Gastroenterology, Hepatology & Nutrition Division at Sheikh Khalifa Medical City (SKMC), a tertiary care center in Abu Dhabi in the UAE, described these initial findings in pediatric intestinal failure patients with crofelemer treatment.
A recognized leader in pediatric gastroenterology, Dr. Miqdady is Professor of Pediatric Gastroenterology at Khalifa University's medical school, and completed his Fellowship in Pediatric Gastroenterology at Baylor College of Medicine and Texas Children's Hospital in Houston. He is a member of Napo's Scientific Advisory Board.
MVID is a devastating ultrarare pediatric disorder, with an estimated worldwide prevalence of 100-200 patients, characterized by severe malabsorption that requires life-sustaining parenteral support to meet the nutritional, fluid and electrolyte requirements of the child, and for which there are currently no approved treatments. MVID has a lethal natural history along with significant co-morbidities. Short bowel syndrome (SBS) affects approximately 10,000 to 20,000 people in the U.S., according to the Crohn's & Colitis Foundation, and it is estimated that the population of SBS patients in Europe is approximately the same size.
In addition to supporting the IIT in the UAE, Napo is also conducting the placebo-controlled clinical trial of crofelemer in pediatric MVID patients at sites in the U.S., E.U., and Middle East under appropriate regulatory approvals in each of these geographies. The company is also providing crofelemer powder for oral solution for use in two expanded access programs in intestinal failure patients the U.S. to treat adult and pediatric intestinal failure patients with short bowel syndrome and MVID, respectively.
The mission of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) is to be a world leader in research, education, clinical practice and advocacy for pediatric gastroenterology, hepatology and nutrition in health and disease. NASPGHAN strives to improve the care of infants, children and adolescents with digestive disorders by promoting advances in clinical care, research and education.
About the Jaguar Health Family of Companies
Jaguar Health, Inc. (Jaguar) is a commercial stage pharmaceuticals company focused on developing novel proprietary prescription medicines sustainably derived from plants from rainforest areas for people and animals with gastrointestinal distress. Jaguar family companies Napo Pharmaceuticals (Napo) and Napo Therapeutics S.p.A. focus on the development and commercialization of novel crofelemer powder for oral solution for the treatment of rare and orphan gastrointestinal disorders with intestinal failure, including microvillus inclusion disease and short bowel syndrome.
For more information about:
Jaguar Health, visit https://jaguar.health
Napo Pharmaceuticals, visit www.napopharma.com
Napo Therapeutics, visit napotherapeutics.com
Forward-Looking Statements
Certain statements in this press release constitute "forward-looking statements." These include statements regarding Jaguar's expectation that crofelemer can potentially extend and save lives of MVID patients, reducing the volume of the PS necessary for them to survive. In some cases, you can identify forward-looking statements by terms such as "may," "will," "should," "expect," "plan," "aim," "anticipate," "could," "intend," "target," "project," "contemplate," "believe," "estimate," "predict," "potential" or "continue" or the negative of these terms or other similar expressions. The forward-looking statements in this release are only predictions. Jaguar has based these forward-looking statements largely on its current expectations and projections about future events. These forward-looking statements speak only as of the date of this release and are subject to several risks, uncertainties, and assumptions, some of which cannot be predicted or quantified and some of which are beyond Jaguar's control. Except as required by applicable law, Jaguar does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Contact:
[email protected]
Jaguar-JAGX
SOURCE: Jaguar Health, Inc.
View the original press release on ACCESS Newswire
L.Miller--AMWN


