
-
 Scotland thrash Tonga in Autumn Nations finale
Scotland thrash Tonga in Autumn Nations finale
-
Three key Irish takeaways from Autumn Nations Series

-
 Imperious Shiffrin swoops to 103rd win at Gurgl
Imperious Shiffrin swoops to 103rd win at Gurgl
-
Schmidt challenges Wallabies to 'roll up their sleeves' after gruesome year

-
 Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva
Washington seeking to 'iron out' Trump proposal details with Ukrainians in Geneva
-
South African centurion Muthusamy celebrates 'awesome' Test journey

-
 Brazil 'very concerned' about US naval build-up near Venezuela
Brazil 'very concerned' about US naval build-up near Venezuela
-
Liverpool a 'mess' says Van Dijk

-
 First blind women's T20 cricket World Cup boosts sport
First blind women's T20 cricket World Cup boosts sport
-
France eye Dupont boost for Six Nations defence

-
 McLaren boss apologises to Norris, Piastri for Vegas disqualification
McLaren boss apologises to Norris, Piastri for Vegas disqualification
-
G20 grapples with splintering world order

-
 Verstappen wins big in Vegas with McLarens disqualified
Verstappen wins big in Vegas with McLarens disqualified
-
Muthusamy, Jansen put South Africa on top in second India Test

-
 Rubio lands in Geneva for talks on Ukraine plan
Rubio lands in Geneva for talks on Ukraine plan
-
Norris and Piastri disqualified from Las Vegas GP

-
 Slovenia holds crunch vote on contested assisted dying law
Slovenia holds crunch vote on contested assisted dying law
-
Aonishiki beomes first Ukrainian to win sumo tournament

-
 Holders Australia drawn with New Zealand in Rugby League World Cup
Holders Australia drawn with New Zealand in Rugby League World Cup
-
Vietnam flooding kills at least 90

-
 Muthusamy's maiden Test century powers South Africa to 428-7
Muthusamy's maiden Test century powers South Africa to 428-7
-
Myanmar junta says nearly 1,600 foreigners arrested in scam hub raids

-
 US signals room for negotiation on Ukraine plan ahead of talks
US signals room for negotiation on Ukraine plan ahead of talks
-
Verstappen wins Las Vegas F1 Grand Prix, Norris edges closer to crown

-
 Muthusamy anchors South Africa to 316-6 in second India Test
Muthusamy anchors South Africa to 316-6 in second India Test
-
Vietnam flood death toll rises to 90

-
 US denies pushing Russian 'wish list' as Ukraine plan
US denies pushing Russian 'wish list' as Ukraine plan
-
Harden's 55 leads Clippers win as Pistons streak hits 12

-
 Kim's first top-10 in 14 years as Ballester wins maiden pro title
Kim's first top-10 in 14 years as Ballester wins maiden pro title
-
Gotham crowned NWSL champions after Lavelle breaks Spirit
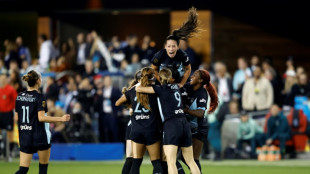
-
 Trump signals room for negotiation on Ukraine plan ahead of talks
Trump signals room for negotiation on Ukraine plan ahead of talks
-
Head shapes up as solution for Australia's opening woes

-
 Tomorrowland bets on Chinese dance music fans with first indoor event
Tomorrowland bets on Chinese dance music fans with first indoor event
-
England slammed as 'brainless' after first Ashes Test capitulation

-
 Slovenia to hold new vote on contested assisted dying law
Slovenia to hold new vote on contested assisted dying law
-
10 Benefits of Choosing Dental Implants After an Extraction

-
 SKYLINE Announces Q3 2025 Financial Results
SKYLINE Announces Q3 2025 Financial Results
-
'Beer tastes better' for Eramsus after win over Irish

-
 No.1 Jeeno leads by six at LPGA Tour Championship
No.1 Jeeno leads by six at LPGA Tour Championship
-
Neres double fires Napoli top in Italy

-
 Bielle-Biarrey masterclass helps France hold off Australia
Bielle-Biarrey masterclass helps France hold off Australia
-
Pogba returns in Monaco loss as PSG stay top in France

-
 COP30: Key reactions to climate deal
COP30: Key reactions to climate deal
-
What did countries agree to at COP30?

-
 Harden's club-record 55 points leads Clippers over Hornets
Harden's club-record 55 points leads Clippers over Hornets
-
Amazon climate deal a 'win' for global unity but fossil fuels untouched

-
 Boos, blowups and last-minute pause as a chaotic COP30 closes out
Boos, blowups and last-minute pause as a chaotic COP30 closes out
-
Farrell proud of Ireland after 'mad' Test with South Africa

-
 Gaza civil defence says 21 killed in Israeli strikes
Gaza civil defence says 21 killed in Israeli strikes
-
South Africa beat ill-disciplined Irish to end Dublin drought


Tivic Secures Barda Meeting for Entolimod(TM) for Acute Radiation Syndrome
Meeting with Radiological and Nuclear Medical Countermeasures Program
FREMONT, CA / ACCESS Newswire / November 18, 2025 / Tivic Health® Systems, Inc. (NASDAQ:TIVC), a late-stage therapeutics company, today announced it has secured an exclusive Techwatch meeting with the Biomedical Advanced Research and Development Authority (BARDA)'s Radiological and Nuclear Medical Countermeasures Program staff.
Tivic leadership will be presenting clinical data on Entolimod's effects on radiation-induced injury and acute radiation syndrome (ARS), its progress on manufacturing readiness and the preparations it has been making for a biologics license application, or BLA.
The Techwatch format allows Tivic and BARDA staff to exchange technical information, establish program priorities, and discuss development pathways for Entolimod to potentially be deployed in mass-casualty, stockpile, and field deployment situations. The process enables BARDA to evaluate technology readiness, understand manufacturing and approval requirements, and identify potential pathways to funding and purchasing.
"This interaction supports Tivic's strategy to develop high-value therapeutic assets with potential government partnership or acquisition pathways," said Jennifer Ernst, CEO of Tivic Health. "We believe our focus on radiation injury and host-directed countermeasures positions us well in a space with defined federal demand and long-term strategic relevance."
Entolimod, Tivic's lead drug candidate, is a novel TLR5 agonist that triggers NF-kB signaling, activating anti-apoptotic and cell protective mechanisms. Under the FDA's Animal Rule, Entolimod for ARS has been the subject of extensive trials and has demonstrated robust survival, enhanced GI tract recovery and improved hematopoiesis in animal models when administered after exposure to ionizing radiation. Clinical studies also suggest Entolimod may serve as a radioprotective agent that can be used prophylactically (prior to exposure) to reduce damage. Entolimod for ARS has been granted Fast Track and Orphan Drug designations by the FDA.
More About BARDA
BARDA is part of the Administration for Strategic Preparedness and Response (ASPR) and operates within the U.S. Department of Health and Human Services (HHS) agency to develop, procure, and stockpile medical countermeasures, including vaccines, drugs and diagnostics that address chemical, biologic, radiologic, and nuclear public health threats.
About Tivic Health Systems, Inc.
Tivic's mission is to harness the immune system to improve clinical outcomes and save lives.
Tivic's biologics compounds activate an innate immune pathway to prevent cell death in the bone marrow and epithelial tissues across systems impacted by radiation and age. The company's lead drug candidate, Entolimod™ for acute radiation syndrome, is a novel TLR5 agonist that has been granted Fast Track and Orphan Drug designations and is in late-stage development. The company also holds rights to Entolimod for the treatment of neutropenia and to Entolasta, an immunologically optimized variant of Entolimod for chronic applications.
Tivic's bioelectronic program has been developing a novel, non-invasive medical device designed to target the neural pathways implicated in many prevalent and debilitating diseases. Early trials show promising signals that Tivic's approach may regulate specific biologic responses, and the company believes its early-stage vagus nerve stimulation device has the potential to deliver clinical outcomes similar to or better than those of surgically implanted devices. To learn more about Tivic, visit: https://ir.tivichealth.com.
Forward-Looking Statements
This press release may contain "forward-looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will," "would," or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on Tivic Health Systems Inc.'s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate, including as a result of interactions with and guidance from the FDA, BARDA, and other regulatory authorities; changes to the company's relationship with its partners; timing and success of clinical trials and study results; the failure to obtain FDA or similar clearances or approvals and noncompliance with FDA or similar regulations; the company's future development of its ncVNS treatment, Entolimod and Entolasta; changes to the company's business strategy; regulatory requirements and pathways for approval; the company's ability to successfully commercialize its product candidates in the future; the potential opportunities that may be available to the company and its product candidates in the future; the company's need for, and ability to secure when needed, additional working capital; the company's ability to continue as a going concern; the company's ability to maintain its Nasdaq listing; and changes in tariffs, inflation, legal, regulatory, political and economic risks. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors. Accordingly, you are cautioned not to place undue reliance on such forward-looking statements. For a discussion of risks and uncertainties relevant to the company, and other important factors, see Tivic Health's filings with the SEC, including, its Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 21, 2025, under the heading "Risk Factors," as well as the company's subsequent filings with the SEC. Forward-looking statements contained in this press release are made as of this date, and Tivic Health Systems, Inc. undertakes no duty to update such information except as required by applicable law.
To learn more about Tivic, visit: https://ir.tivichealth.com
Investor Contact:
Hanover International, Inc.
[email protected]
Media Contact:
Deanne Eagle or Laura Min Jackson
[email protected]
SOURCE: Tivic Health Systems
View the original press release on ACCESS Newswire
C.Garcia--AMWN


