
-
 Apple earnings soar as China iPhone sales surge
Apple earnings soar as China iPhone sales surge
-
Forest, Celtic head into Europa League play-offs as Villa win

-
 With Trump administration watching, Canada oil hub faces separatist bid
With Trump administration watching, Canada oil hub faces separatist bid
-
What are the key challenges awaiting the new US Fed chair?

-
 Trump's new Minneapolis point man vows 'smarter' operation
Trump's new Minneapolis point man vows 'smarter' operation
-
Trump says Putin to halt Kyiv strikes for week amid harsh cold

-
 De Kock ton clinches T20 series for South Africa against West Indies
De Kock ton clinches T20 series for South Africa against West Indies
-
Chiles's appeal to retain Olympic bronze sent back to CAS

-
 Iran threatens to hit US bases and carriers in event of attack
Iran threatens to hit US bases and carriers in event of attack
-
If not now, when? LeBron tears stoke retirement talk

-
 Ex-OPEC president denies bribe-taking at London corruption trial
Ex-OPEC president denies bribe-taking at London corruption trial
-
Another Arctic blast bears down on US as snow cleanup drags on

-
 Iran's IRGC: the feared 'Pasdaran' behind deadly crackdown
Iran's IRGC: the feared 'Pasdaran' behind deadly crackdown
-
Israeli settler leader lauds Jewish prayer at contested West Bank tomb

-
 Trump says Putin agreed not to attack freezing Kyiv for a week
Trump says Putin agreed not to attack freezing Kyiv for a week
-
Moscow records heaviest snowfall in over 200 years

-
 Polar bears bulk up despite melting Norwegian Arctic: study
Polar bears bulk up despite melting Norwegian Arctic: study
-
Waymo gears up to launch robotaxis in London this year

-
 Colombia restricts import of drones used in explosives attacks
Colombia restricts import of drones used in explosives attacks
-
French IT group Capgemini under fire over ICE links

-
 Oil jumps on Trump's Iran threat; gold retreats from highs
Oil jumps on Trump's Iran threat; gold retreats from highs
-
Melania Trump premieres multi-million-dollar documentary

-
 Holders PSG, Real Madrid among clubs awaiting Champions League play-offs draw
Holders PSG, Real Madrid among clubs awaiting Champions League play-offs draw
-
England look to fine tune for T20 World Cup with Sri Lanka series

-
 US Senate vote to avert government shutdown expected to fail
US Senate vote to avert government shutdown expected to fail
-
Colombian president angers churches with Jesus sex comments

-
 Turkey to offer mediation in US-Iran showdown
Turkey to offer mediation in US-Iran showdown
-
World Cup skiing returns to Crans-Montana after deadly fire

-
 EU designates Iran Guards as 'terrorist organisation'
EU designates Iran Guards as 'terrorist organisation'
-
Czechs wind up black coal mining in green energy switch

-
 Where does Iraq stand as US turns up heat on Iran?
Where does Iraq stand as US turns up heat on Iran?
-
Vietnam designer makes history as Paris Haute Couture wraps up

-
 Oil jumps, gold climbs further on Trump's Iran threat
Oil jumps, gold climbs further on Trump's Iran threat
-
Denmark hails 'very constructive' meeting with US over Greenland

-
 Phan Huy: the prodigy putting Vietnam on the fashion map
Phan Huy: the prodigy putting Vietnam on the fashion map
-
US border chief says not 'surrendering' immigration mission

-
 EU to put Iran Guards on 'terrorist list'
EU to put Iran Guards on 'terrorist list'
-
Pegula calls herself 'shoddy, erratic' in Melbourne semi-final loss

-
 All hands on deck: British Navy sobers up alcohol policy
All hands on deck: British Navy sobers up alcohol policy
-
Irish Six Nations hopes hit by Aki ban

-
 Britain's Starmer hails 'good progress' after meeting China's Xi
Britain's Starmer hails 'good progress' after meeting China's Xi
-
Parrots rescued as landslide-hit Sicilian town saves pets

-
 Gold surges further, oil jumps on Trump's Iran threat
Gold surges further, oil jumps on Trump's Iran threat
-
No handshake as Sabalenka sets up repeat of 2023 Melbourne final

-
 Iran's IRGC: the feared 'Pasdaran' set for EU terror listing
Iran's IRGC: the feared 'Pasdaran' set for EU terror listing
-
EU eyes migration clampdown with push on deportations, visas

-
 Rybakina battles into Australian Open final against Sabalenka
Rybakina battles into Australian Open final against Sabalenka
-
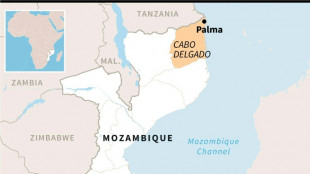
Giant Mozambique gas project resumes after 5-year security suspension
-
 Iran vows 'crushing response', EU targets Revolutionary Guards
Iran vows 'crushing response', EU targets Revolutionary Guards
-
Northern Mozambique: massive gas potential in an insurgency zone


Altamira Therapeutics Announces Market Approval of Bentrio Nasal Spray in China
Regulatory clearance of Bentrio in allergic rhinitis for Mainland China
Nearly 200 million Chinese estimated to suffer from allergic rhinitis
Bentrio to be launched by partner Nuance Pharma
HAMILTON, BERMUDA / ACCESS Newswire / December 19, 2025 / Altamira Therapeutics Ltd. ("Altamira" or the "Company") (OTCID:CYTOF), a company dedicated to developing and supplying nucleic acid delivery technology for partners in biotech and pharma, today announced that its associate company Altamira Medica ("Medica") has obtained marketing approval from the National Medical Products Administration (NMPA) of China for its Bentrio® nasal spray in allergic rhinitis. The clearance will allow Nuance Pharma, Medica's licensee and distribution partner, to start marketing and distributing Bentrio in Mainland China. According to epidemiological studies, nearly 200 million people in China are estimated to be affected by allergic rhinitis, and prevalence keeps increasing. [1]
"We are very excited by the regulatory clearance of Bentrio for China, which is one of the world's largest markets for 'over the counter' consumer health products," commented Thomas Meyer, Medica's Chairman and CEO. "Thanks to its triple mode of action, which does not require any drug ingredient for protecting against airborne allergens, and its preservative-free formulation, Bentrio has demonstrated strong efficacy and good tolerability across several allergic rhinitis trials. We look forward to supporting the launch of the product in China by our esteemed partner Nuance Pharma, which has built a strong and growing franchise in consumer health and in respiratory diseases."
"The regulatory approval of Bentrio represents an important milestone for Nuance Pharma's effort in the allergy space" commented Mark G. Lotter, CEO and Founder of Nuance Pharma. "Building upon Nuance's existing efforts on Bentrio in the region and commercial capabilities in China, we are excited to broaden the access of Bentrio to benefit the allergic rhinitis patients in China."
About Bentrio
Bentrio is an "over the counter" drug-free nasal spray for personal protection against airborne allergens and, where approved, against airborne viruses. Upon application into the nose, Bentrio forms a protective gel layer on the nasal mucosa. This thin film is designed to prevent the contact of airborne particles with cells; in addition, the composition serves to bind such particles and help with their discharge. The efficacy and safety of Bentrio have been demonstrated in a total of four clinical trials, of which the largest one ("NASAR" study) enrolled 100 patients suffering from seasonal allergic rhinitis. In NASAR, participants self-administered either Bentrio or saline nasal spray for two weeks 3 times per day. The study showed a statistically significant reduction in the mean daily reflective Total Nasal Symptom Score (rTNSS) for Bentrio compared to saline (p = 0.013), as well as a statistically highly significant improvement in health-related quality of life (Rhinoconjunctivitis Quality of Life Questionnaire, p www.bentrio.com
About Altamira Therapeutics
Altamira Therapeutics is developing and supplying peptide-based nanoparticle technologies for efficient nucleic acid delivery (xPhore™ platform). The versatile delivery platform is suited for different nucleic acid modalities, including siRNA, mRNA, circRNA, as well as DNA, and made available to pharma or biotech companies through out-licensing. The Company has two proprietary flagship programs based on xPhore and siRNA payloads: AM-401 for KRAS driven cancer and AM-411 for rheumatoid arthritis, both in preclinical development beyond in vivo proof of concept. In addition, Altamira holds a 49% stake (with additional economic rights) in Altamira Medica AG, which owns its commercial-stage legacy asset Bentrio, an OTC nasal spray for allergic rhinitis. Further, the Company is in the process of partnering / divesting its inner ear legacy assets. Founded in 2003, Altamira is headquartered in Hamilton, Bermuda, with its main operations in Basel, Switzerland. For more information, visit: https://altamiratherapeutics.com/
About Nuance Pharma
Nuance Pharma is an innovation focused biopharmaceutical company, with both late-stage clinical pipeline and commercial stage asset portfolio. Focusing on specialty care, Nuance has established a differentiated combination of commercialized assets and innovative pipeline across respiratory, allergy, emergency care and iron deficiency anemia. With the mission to address critical unmet medical needs in Asia Pacific, Nuance deploys the Dual Wheel model that develops a global leading innovative pipeline, while maintaining a self-sustainable commercial operation in both China and Asia as a region. For more information, visit: https://www.nuancepharma.com/
Forward-Looking Statements
This press release may contain statements that constitute "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements are statements other than historical facts and may include statements that address future operating, financial or business performance or Altamira's strategies or expectations. In some cases, you can identify these statements by forward-looking words such as "may", "might", "will", "should", "expects", "plans", "anticipates", "believes", "estimates", "predicts", "projects", "potential", "outlook" or "continue", or the negative of these terms or other comparable terminology. Forward-looking statements are based on management's current expectations and beliefs and involve significant risks and uncertainties that could cause actual results, developments and business decisions to differ materially from those contemplated by these statements. These risks and uncertainties include but are not limited to the clinical utility of Altamira's product candidates, the timing or likelihood of regulatory filings and approvals, Altamira's intellectual property position and Altamira's financial position. These risks and uncertainties also include, but are not limited to, those described under the caption "Risk Factors" in Altamira's Annual Report on Form 20-F for the year ended December 31, 2024, and in Altamira's other filings with the Securities Exchange Commission ("SEC"), which are available free of charge on the SEC's website at: www.sec.gov. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those indicated. All forward-looking statements and all subsequent written and oral forward-looking statements attributable to Altamira or to persons acting on behalf of Altamira are expressly qualified in their entirety by reference to these risks and uncertainties. You should not place undue reliance on forward-looking statements. Forward-looking statements speak only as of the date they are made, and Altamira does not undertake any obligation to update them in light of new information, future developments or otherwise, except as may be required under applicable law.
Investor Contact:
[1] Zhang Y, Zhang L (2014), Prevalence of Allergic Rhinitis in China, Allergy Asthma Immunol Res. 6(2):105-13, https://e-aair.org/DOIx.php?id=10.4168/aair.2014.6.2.105
SOURCE: Altamira Therapeutics Ltd.
View the original press release on ACCESS Newswire
A.Mahlangu--AMWN
