
-
 If not now, when? LeBron tears stoke retirement talk
If not now, when? LeBron tears stoke retirement talk
-
Ex-OPEC president denies bribe-taking at London corruption trial

-
 Another Arctic blast bears down on US as snow cleanup drags on
Another Arctic blast bears down on US as snow cleanup drags on
-
Iran's IRGC: the feared 'Pasdaran' behind deadly crackdown

-
 Israeli settler leader lauds Jewish prayer at contested West Bank tomb
Israeli settler leader lauds Jewish prayer at contested West Bank tomb
-
Trump says Putin agreed not to attack freezing Kyiv for a week

-
 Moscow records heaviest snowfall in over 200 years
Moscow records heaviest snowfall in over 200 years
-
Polar bears bulk up despite melting Norwegian Arctic: study

-
 Waymo gears up to launch robotaxis in London this year
Waymo gears up to launch robotaxis in London this year
-
Colombia restricts import of drones used in explosives attacks

-
 French IT group Capgemini under fire over ICE links
French IT group Capgemini under fire over ICE links
-
Oil jumps on Trump's Iran threat; gold retreats from highs

-
 Melania Trump premieres multi-million-dollar documentary
Melania Trump premieres multi-million-dollar documentary
-
Holders PSG, Real Madrid among clubs awaiting Champions League play-offs draw

-
 England look to fine tune for T20 World Cup with Sri Lanka series
England look to fine tune for T20 World Cup with Sri Lanka series
-
US Senate vote to avert government shutdown expected to fail

-
 Colombian president angers churches with Jesus sex comments
Colombian president angers churches with Jesus sex comments
-
Turkey to offer mediation in US-Iran showdown

-
 World Cup skiing returns to Crans-Montana after deadly fire
World Cup skiing returns to Crans-Montana after deadly fire
-
EU designates Iran Guards as 'terrorist organisation'

-
 Czechs wind up black coal mining in green energy switch
Czechs wind up black coal mining in green energy switch
-
Where does Iraq stand as US turns up heat on Iran?

-
 Vietnam designer makes history as Paris Haute Couture wraps up
Vietnam designer makes history as Paris Haute Couture wraps up
-
Oil jumps, gold climbs further on Trump's Iran threat

-
 Denmark hails 'very constructive' meeting with US over Greenland
Denmark hails 'very constructive' meeting with US over Greenland
-
Phan Huy: the prodigy putting Vietnam on the fashion map

-
 US border chief says not 'surrendering' immigration mission
US border chief says not 'surrendering' immigration mission
-
EU to put Iran Guards on 'terrorist list'

-
 Pegula calls herself 'shoddy, erratic' in Melbourne semi-final loss
Pegula calls herself 'shoddy, erratic' in Melbourne semi-final loss
-
All hands on deck: British Navy sobers up alcohol policy

-
 Irish Six Nations hopes hit by Aki ban
Irish Six Nations hopes hit by Aki ban
-
Britain's Starmer hails 'good progress' after meeting China's Xi

-
 Parrots rescued as landslide-hit Sicilian town saves pets
Parrots rescued as landslide-hit Sicilian town saves pets
-
Gold surges further, oil jumps on Trump's Iran threat

-
 No handshake as Sabalenka sets up repeat of 2023 Melbourne final
No handshake as Sabalenka sets up repeat of 2023 Melbourne final
-
Iran's IRGC: the feared 'Pasdaran' set for EU terror listing

-
 EU eyes migration clampdown with push on deportations, visas
EU eyes migration clampdown with push on deportations, visas
-
Rybakina battles into Australian Open final against Sabalenka

-
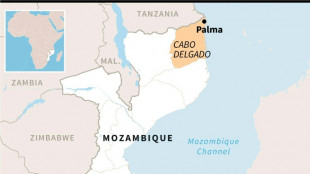 Giant Mozambique gas project resumes after 5-year security suspension
Giant Mozambique gas project resumes after 5-year security suspension
-
Iran vows 'crushing response', EU targets Revolutionary Guards

-
 Northern Mozambique: massive gas potential in an insurgency zone
Northern Mozambique: massive gas potential in an insurgency zone
-
Gold demand hits record high on Trump policy doubts: industry

-
 Show must go on: London opera chief steps in for ailing tenor
Show must go on: London opera chief steps in for ailing tenor
-
UK drugs giant AstraZeneca announces $15 bn investment in China

-
 US scrutiny of visitors' social media could hammer tourism: trade group
US scrutiny of visitors' social media could hammer tourism: trade group
-
'Watch the holes'! Paris fashion crowd gets to know building sites

-
 Power, pace and financial muscle: How Premier League sides are ruling Europe
Power, pace and financial muscle: How Premier League sides are ruling Europe
-
'Pesticide cocktails' pollute apples across Europe: study

-
 Ukraine's Svitolina feels 'very lucky' despite Australian Open loss
Ukraine's Svitolina feels 'very lucky' despite Australian Open loss
-
Money laundering probe overshadows Deutsche Bank's record profits


Trump’s Marijuana Schedule III Order Launches a Federally Regulated Healthcare Sector
MMJ's position is further fortified by its FDA Orphan Drug Designations for the treatment of Huntington's Disease. These designations are awarded to drugs intended for the safe and effective treatment of rare diseases and provide MMJ with critical incentives, including: Seven Years of Market Exclusivity: Protecting MMJ's innovations upon FDA approval. Tax Credits: Applied to qualified clinical testing expenses. Waived FDA Fees: Significantly reducing the capital barriers to drug commercialization. Accelerated Review: Streamlining the path to provide relief to patients with unmet medical needs.
WASHINGTON, D.C. / ACCESS Newswire / December 22, 2025 / MMJ International Holdings, MMJ BioPharma Cultivation and MMJ BioPharma Labs ("MMJ"), a pioneer in pharmaceutical-grade cannabinoid drug development, today issued a statement following President Donald Trump's historic executive order directing the reclassification of cannabis from Schedule I to Schedule III under the Controlled Substances Act. The order marks a decisive shift in federal policy-formally recognizing cannabis' credible therapeutic value and validating a science-first, FDA-regulated pharmaceutical pathway that MMJ has pursued for years.

For decades, Schedule I classification functioned as a structural barrier to legitimate clinical research, limiting FDA oversight and leaving millions of patients- including nearly one in four U.S. adults living with chronic pain-without access to standardized, federally regulated treatment options. President Trump's action dismantles that barrier and aligns federal drug policy with established medical and clinical reality.
A Strategic Advantage in a Schedule III Landscape
The transition to Schedule III draws a clear line between recreational commerce and pharmaceutical medicine. MMJ enters this new landscape with a substantial strategic advantage, having already completed much of the foundational work required for pharmaceutical legitimacy, including:
Advanced Formulation - Development of a proprietary, pharmaceutical-grade soft-gel capsule
Scientific Validation - Completion of comprehensive stability testing and chromatography
Clinical Readiness - Advancement of FDA-authorized clinical programs targeting chronic pain and neurological disorders
This infrastructure positions MMJ to move immediately as regulatory friction eases.
Focus Shifts to DOJ Implementation
With federal policy now clarified, attention turns to the U.S. Department of Justice and Attorney General Pamela Bondi, whose actions will determine the speed at which lawful, FDA-authorized cannabinoid research proceeds. MMJ's prior constitutional challenges to the DEA's administrative process-challenges that resulted in DOJ acknowledgments of fundamental defects in the former framework-underscore the importance of prompt, science-driven implementation.
MMJ urges the Department of Justice to ensure that FDA-authorized programs advance without further delay so patients can access safe, consistent, and reproducible medicines under federal oversight.
As institutional capital evaluates the implications of Schedule III, the distinction between speculative cannabis retail and regulated cannabinoid medicine has never been clearer. MMJ's early and sustained investment in pharmaceutical manufacturing, clinical validation, and regulatory compliance offers investors exposure to what is now unmistakably a healthcare sector.
"The federal government has caught up to the strategy MMJ has pursued since its inception," the company added. "The question is no longer whether cannabis has medical value, but how quickly pharmaceutical-grade solutions can reach patients."
About MMJ International Holdings
MMJ International Holdings is a biopharmaceutical company focused on the FDA-authorized development of cannabinoid-based medicines. Through its subsidiaries, the company advances pharmaceutical-grade, reproducible final dosage forms supported by clinical trials and high-standard manufacturing for the treatment of chronic pain and neurological disorders.
MMJ is represented by attorney Megan Sheehan.
CONTACT:
Madison Hisey
[email protected]
203-231-8583
SOURCE: MMJ International Holdings
View the original press release on ACCESS Newswire
F.Pedersen--AMWN