
-
 If not now, when? LeBron tears stoke retirement talk
If not now, when? LeBron tears stoke retirement talk
-
Ex-OPEC president denies bribe-taking at London corruption trial

-
 Another Arctic blast bears down on US as snow cleanup drags on
Another Arctic blast bears down on US as snow cleanup drags on
-
Iran's IRGC: the feared 'Pasdaran' behind deadly crackdown

-
 Israeli settler leader lauds Jewish prayer at contested West Bank tomb
Israeli settler leader lauds Jewish prayer at contested West Bank tomb
-
Trump says Putin agreed not to attack freezing Kyiv for a week

-
 Moscow records heaviest snowfall in over 200 years
Moscow records heaviest snowfall in over 200 years
-
Polar bears bulk up despite melting Norwegian Arctic: study

-
 Waymo gears up to launch robotaxis in London this year
Waymo gears up to launch robotaxis in London this year
-
Colombia restricts import of drones used in explosives attacks

-
 French IT group Capgemini under fire over ICE links
French IT group Capgemini under fire over ICE links
-
Oil jumps on Trump's Iran threat; gold retreats from highs

-
 Melania Trump premieres multi-million-dollar documentary
Melania Trump premieres multi-million-dollar documentary
-
Holders PSG, Real Madrid among clubs awaiting Champions League play-offs draw

-
 England look to fine tune for T20 World Cup with Sri Lanka series
England look to fine tune for T20 World Cup with Sri Lanka series
-
US Senate vote to avert government shutdown expected to fail

-
 Colombian president angers churches with Jesus sex comments
Colombian president angers churches with Jesus sex comments
-
Turkey to offer mediation in US-Iran showdown

-
 World Cup skiing returns to Crans-Montana after deadly fire
World Cup skiing returns to Crans-Montana after deadly fire
-
EU designates Iran Guards as 'terrorist organisation'

-
 Czechs wind up black coal mining in green energy switch
Czechs wind up black coal mining in green energy switch
-
Where does Iraq stand as US turns up heat on Iran?

-
 Vietnam designer makes history as Paris Haute Couture wraps up
Vietnam designer makes history as Paris Haute Couture wraps up
-
Oil jumps, gold climbs further on Trump's Iran threat

-
 Denmark hails 'very constructive' meeting with US over Greenland
Denmark hails 'very constructive' meeting with US over Greenland
-
Phan Huy: the prodigy putting Vietnam on the fashion map

-
 US border chief says not 'surrendering' immigration mission
US border chief says not 'surrendering' immigration mission
-
EU to put Iran Guards on 'terrorist list'

-
 Pegula calls herself 'shoddy, erratic' in Melbourne semi-final loss
Pegula calls herself 'shoddy, erratic' in Melbourne semi-final loss
-
All hands on deck: British Navy sobers up alcohol policy

-
 Irish Six Nations hopes hit by Aki ban
Irish Six Nations hopes hit by Aki ban
-
Britain's Starmer hails 'good progress' after meeting China's Xi

-
 Parrots rescued as landslide-hit Sicilian town saves pets
Parrots rescued as landslide-hit Sicilian town saves pets
-
Gold surges further, oil jumps on Trump's Iran threat

-
 No handshake as Sabalenka sets up repeat of 2023 Melbourne final
No handshake as Sabalenka sets up repeat of 2023 Melbourne final
-
Iran's IRGC: the feared 'Pasdaran' set for EU terror listing

-
 EU eyes migration clampdown with push on deportations, visas
EU eyes migration clampdown with push on deportations, visas
-
Rybakina battles into Australian Open final against Sabalenka

-
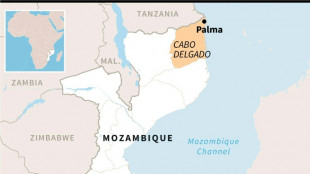 Giant Mozambique gas project resumes after 5-year security suspension
Giant Mozambique gas project resumes after 5-year security suspension
-
Iran vows 'crushing response', EU targets Revolutionary Guards

-
 Northern Mozambique: massive gas potential in an insurgency zone
Northern Mozambique: massive gas potential in an insurgency zone
-
Gold demand hits record high on Trump policy doubts: industry

-
 Show must go on: London opera chief steps in for ailing tenor
Show must go on: London opera chief steps in for ailing tenor
-
UK drugs giant AstraZeneca announces $15 bn investment in China

-
 US scrutiny of visitors' social media could hammer tourism: trade group
US scrutiny of visitors' social media could hammer tourism: trade group
-
'Watch the holes'! Paris fashion crowd gets to know building sites

-
 Power, pace and financial muscle: How Premier League sides are ruling Europe
Power, pace and financial muscle: How Premier League sides are ruling Europe
-
'Pesticide cocktails' pollute apples across Europe: study

-
 Ukraine's Svitolina feels 'very lucky' despite Australian Open loss
Ukraine's Svitolina feels 'very lucky' despite Australian Open loss
-
Money laundering probe overshadows Deutsche Bank's record profits


CASI Pharmaceuticals Receives Extension from Nasdaq to Meet Listing Requirements
SOUTH SAN FRANCISCO, CALIFORNIA / ACCESS Newswire / December 23, 2025 / CASI Pharmaceuticals, Inc. (NASDAQ:CASI), a clinical-stage biopharmaceutical company focused on developing CID-103, a potential best-in-class, anti-CD38 monoclonal antibody for patients with organ transplant rejection and autoimmune diseases, today announced the Nasdaq Hearings Panel (the "Panel") has granted CASI's request to continued listing on the Nasdaq Stock Market (the "Nasdaq") subject to the Company regaining compliance with the Market Value of Listed Securities (MVLS) Rule 5550(b)(2) by February 17, 2026.
The Panel granted the extension after reviewing the recent efforts made by the Company, including the appointments of an experienced Chief Executive Officer and a new Non-Executive Chairman, a commitment to focus on execution of CID-103 development, execution of a convertible note purchase agreement, additional financing initiatives underway, and planned divestiture of China assets.
As previously reported, on May 5, 2025, Nasdaq notified the Company that its market value of listed securities (MVLS) had fallen below the minimum requirement of $35 million for 30 consecutive trading days and as a result, did not comply with Listing Rule 5550(b)(2). The Company was provided 180 calendar days, or until November 3, 2025, to regain compliance with this rule.
CASI appealed this Determination and was granted a further extension of the grace period to February 17, 2026 to comply with the Nasdaq MVLS requirement. This grace period will stay the suspension of the Company's securities.
CASI remains committed to full compliance with all Nasdaq listing requirements and will take all necessary actions to regain compliance.
About CASI Pharmaceuticals
CASI Pharmaceuticals, Inc. is a public biopharmaceutical company developing CID-103, an anti-CD38 monoclonal antibody for organ transplant rejection and autoimmune diseases.
CID-103 is a fully human IgG1, potentially best-in-class, clinical stage, anti-CD38 monoclonal antibody which targets a unique epitope and has demonstrated an encouraging pre-clinical efficacy and clinical safety profile compared to other anti-CD38 monoclonal antibodies, and for which CASI owns exclusive global rights. CASI received FDA IND clearance to conduct a Phase 1 study in renal allograft antibody-mediated rejection (AMR) in the U.S. and plans for first patient in first quarter of 2026. In parallel, CASI is actively recruiting and dosing patients in an ongoing Phase 1 study in immune thrombocytopenia (ITP). In addition, CASI is assessing multiple technologies for development of a stable, high concentration protein solution for subcutaneous injection.
More information on CASI is available at www.casipharmaceuticals.com.
Forward Looking Statements
This announcement contains forward-looking statements. These statements are made under the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as "will," "expects," "future," "intends," "plans," "believes," and similar statements. Among other things, the business outlook and quotations from management in this announcement, as well as the Company's strategic and operational plans, contain forward-looking statements. The Company may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the "SEC"), in its annual report to shareholders, in press releases and other written materials and in oral statements made by its officers, directors or employees to third parties. Statements that are not historical facts, including statements about the Company's beliefs and expectations, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties. A number of factors could cause actual results to differ materially from those contained in any forward-looking statement, including but not limited to the following: the possibility that we may be delisted from trading on The Nasdaq Capital Market if we are not granted any extension of compliance period; uncertainties related to the possibility that the transaction for the divestiture of certain assets in China (the "Transaction") will not occur as planned if events arise that result in the termination of the Equity and Assets Transfer Agreement, or if one or more of the various closing conditions to the Transaction are not satisfied or waived; the possibility that our plan with respect to our business operations after the consummation of the Transaction can be implemented successfully; our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern; the volatility in the market price of our ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict when or if our product candidates will be approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, PRC National Medical Products Administration, or other regulatory authorities; our inability to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products; and our dependence on third parties; the risks related to our dependence on Juventas to conduct the clinical development of CNCT19 and to partner with us to co-market CNCT19; risks related to our dependence on Juventas to ensure the patent protection and prosecution for CNCT19; the risk related to the Company's ongoing development of and regulatory application for CID-103 with respect to the treatment of antibody-mediated rejection for organ transplant and the license arrangements of CID-103; risks relating to interests of our largest shareholder and our Chairman that differ from our other shareholders; risks related to the development of a new manufacturing facility by CASI Pharmaceuticals (Wuxi) Co., Ltd.; and risks related to our disagreement with Acrotech with respect to the termination of agreements regarding EVOMELA®. Further information regarding these and other risks is included in the Company's filings with the SEC. All information provided herein is as of the date of this announcement, and the Company undertakes no obligation to update any forward-looking statement, except as required under applicable law. We caution readers not to place undue reliance on any forward-looking statements contained herein.
EVOMELA® is proprietary to Acrotech Biopharma Inc. and its affiliates. FOLOTYN® is proprietary to Acrotech Biopharma Inc and its affiliates. The Company is currently involved in disputes and legal proceedings related to certain pipeline products, including EVOMELA® and CNCT-19.Please refer to the Company's earlier SEC filing for further information.
INVESTOR CONTACT:
Ingrid Choong, PhD
650-619-6115
[email protected]
SOURCE: CASI Pharmaceuticals
View the original press release on ACCESS Newswire
Ch.Kahalev--AMWN