
-
 Champions League crunch time as pressure piles on Europe's elite
Champions League crunch time as pressure piles on Europe's elite
-
Harry arrives at London court for latest battle against UK newspaper

-
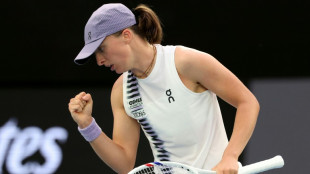 Swiatek survives scare to make Australian Open second round
Swiatek survives scare to make Australian Open second round
-
Over 400 Indonesians 'released' by Cambodian scam networks: ambassador

-
 Europe readying steps against Trump tariff 'blackmail' on Greenland: Berlin
Europe readying steps against Trump tariff 'blackmail' on Greenland: Berlin
-
What is the EU's anti-coercion 'bazooka' it could use against US?

-
 Infantino condemns Senegal for 'unacceptable scenes' in AFCON final
Infantino condemns Senegal for 'unacceptable scenes' in AFCON final
-
Gold, silver hit peaks and stocks sink on new US-EU trade fears

-
 Trailblazer Eala exits Australian Open after 'overwhelming' scenes
Trailblazer Eala exits Australian Open after 'overwhelming' scenes
-
Warhorse Wawrinka stays alive at farewell Australian Open

-
 Bangladesh face deadline over refusal to play World Cup matches in India
Bangladesh face deadline over refusal to play World Cup matches in India
-
High-speed train collision in Spain kills 39, injures dozens

-
 Auger-Aliassime retires in Melbourne heat with cramp
Auger-Aliassime retires in Melbourne heat with cramp
-
Melbourne home hope De Minaur 'not just making up the numbers'

-
 Risking death, Indians mess with the bull at annual festival
Risking death, Indians mess with the bull at annual festival
-
Ghana's mentally ill trapped between prayer and care

-
 UK, France mull social media bans for youth as debate rages
UK, France mull social media bans for youth as debate rages
-
Japan PM to call snap election seeking stronger mandate

-
 Switzerland's Ruegg sprints to second Tour Down Under title
Switzerland's Ruegg sprints to second Tour Down Under title
-
China's Buddha artisans carve out a living from dying trade

-
 Stroking egos key for Arbeloa as Real Madrid host Monaco
Stroking egos key for Arbeloa as Real Madrid host Monaco
-
'I never felt like a world-class coach', says Jurgen Klopp

-
 Ruthless Anisimova races into Australian Open round two
Ruthless Anisimova races into Australian Open round two
-
Australia rest Cummins, Hazlewood, Maxwell for Pakistan T20 series

-
 South Korea, Italy agree to deepen AI, defence cooperation
South Korea, Italy agree to deepen AI, defence cooperation
-
Vietnam begins Communist Party congress to pick leaders

-
 Gauff 'erases' serving wobbles in winning Melbourne start
Gauff 'erases' serving wobbles in winning Melbourne start
-
China's 2025 economic growth among slowest in decades

-
 Gauff, Medvedev through in Australia as Djokovic begins record Slam quest
Gauff, Medvedev through in Australia as Djokovic begins record Slam quest
-
Who said what at 2025 Africa Cup of Nations

-
 Three-time finalist Medvedev grinds into Australian Open round two
Three-time finalist Medvedev grinds into Australian Open round two
-
Auger-Aliassime retires from Melbourne first round with cramp

-
 Rams fend off Bears comeback as Patriots advance in NFL playoffs
Rams fend off Bears comeback as Patriots advance in NFL playoffs
-
Thousands march in US to back Iranian anti-government protesters

-
 Gotterup charges to Sony Open victory in Hawaii
Gotterup charges to Sony Open victory in Hawaii
-
Gold, silver hit records and stocks fall as Trump fans trade fears

-
 Auger-Aliassime retires injured from Melbourne first round
Auger-Aliassime retires injured from Melbourne first round
-
Gauff through, Auger-Aliassime retires as Djokovic begins record quest

-
 China says economy grew 5% last year, among slowest in decades
China says economy grew 5% last year, among slowest in decades
-
Young star Zheng may have to give back Australian Open prize money

-
 Gauff overcomes wobble in winning start to Melbourne title bid
Gauff overcomes wobble in winning start to Melbourne title bid
-
Harry set for final courtroom battle against UK media

-
 'It wasn't clean': Mother mourns son killed in US Maduro assault
'It wasn't clean': Mother mourns son killed in US Maduro assault
-
Louvre heist probe: What we know

-
 Surging billionaire wealth a political threat, Oxfam warns as Davos opens
Surging billionaire wealth a political threat, Oxfam warns as Davos opens
-
Morocco fans stunned, disappointed as Senegal win Africa title

-
 Senegal fuelled by 'injustice' in AFCON final triumph, says hero Gueye
Senegal fuelled by 'injustice' in AFCON final triumph, says hero Gueye
-
Snowline Gold And First Nation of Na-Cho Nyäk Dun Sign Memorandum of Understanding

-
 Battery X Metals Announces Closing of $2.4 Million Private Placement Financing with Participation from Former Director and Executive Officer of Fortune 500 Skechers USA, Inc.
Battery X Metals Announces Closing of $2.4 Million Private Placement Financing with Participation from Former Director and Executive Officer of Fortune 500 Skechers USA, Inc.
-
Apex Summarizes Progress to-date and 2026 Outlook at the Rift Rare Earth Project


BioNxt Reports Successful Final In-Vivo Dosing Study Results Supporting Superior Bioavailability of Cladribine Sublingual ODF
VANCOUVER, BC / ACCESS Newswire / January 19, 2026 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTCQB:BNXTF)(FSE:BXT), a bioscience innovator specializing in advanced drug delivery systems, is pleased to announce that it has completed and received preliminary results from its comparative pharmacokinetics ("PK") large-mass animal study in pigs. The study measured how efficiently cladribine is absorbed into the bloodstream over time and compared BioNxt's proprietary orally dissolving film ("ODF") with the reference tablet formulation, evaluating the Company's swallow-free, sublingual ODF formulation for Multiple Sclerosis (MS).
BioNxt reports that the study outcome was successful and supports superior bioavailability for the Company's cladribine ODF formulation under the study conditions. The Company has initiated a full internal screening and assessment of the final dataset, including calculations and interpretation of key pharmacokinetic parameters. Further information will be provided once BioNxt has completed its comprehensive internal analysis.
"We are extremely encouraged by the successful outcome of this study," said Hugh Rogers, CEO at BioNxt. "The results reinforce our belief that sublingual ODF delivery can materially improve the efficiency of cladribine administration and may ultimately support better patient tolerability and adoption. We look forward to sharing additional details once our complete internal analysis is finalized. The data from this study is critical to optimize the dosing parameters in our upcoming human bioequivalence study."
Study Overview: Comparative Pharmacokinetics Study in Pigs
To evaluate how effectively BioNxt's swallow-free cladribine ODF delivers cladribine into the bloodstream, the Company conducted a comparative crossover pharmacokinetics study in adult pigs, weighing approximately 40-50 kg. This specific breed of pig is widely used in pharmaceutical research as a non-rodent large-mass preclinical model due to their high physiological, anatomical, and metabolic similarities to humans, particularly in drug absorption and systemic exposure.
The objective was to compare BioNxt's cladribine 10 mg orally dissolving film (ODF) with the reference name-brand 10 mg tablet, a commercially established Multiple Sclerosis therapy with annual global sales exceeding USD 1 billion and continued double-digit annual growth, with both formulations administered at a single dose equivalent to 5 mg of cladribine per animal. The reference tablet was administered by oral dosing via gavage, while the ODF was placed directly into the oral cavity to support sublingual absorption.
To track cladribine levels over time, blood samples were collected before dosing and at several time points over 24 hours following administration. This allowed BioNxt to measure key performance indicators such as peak concentration, overall exposure, and how long cladribine remained in the body, supporting a clear assessment of bioavailability.
The study was initiated in November 2025 and completed in December 2025, with the final dataset now received by the Company for internal analysis.
Potential to Reduce Side Effects and Lower Required Active Ingredient
The Company's study results confirm that enhanced bioavailability achieved through sublingual ODF delivery materially improves delivery efficiency compared with conventional oral administration. By enabling more efficient absorption of cladribine into the bloodstream, the ODF approach supports the achievement of therapeutic exposure with lower overall systemic drug exposure, which is a critical factor in managing tolerability for immunomodulatory therapies such as cladribine.
This improved delivery efficiency directly supports BioNxt's strategy to reduce unnecessary systemic burden, creating the foundation for a meaningful reduction in dose-related side effects while maintaining pharmacokinetic performance. In addition, the swallow-free, sublingual ODF format offers a more patient-friendly treatment experience, particularly for patients with swallowing difficulties or sensitivity to conventional oral dosage forms.
BioNxt views these findings as a key validation of its ODF drug-delivery platform and a strategically important step in building a differentiated Multiple Sclerosis therapy profile with strong commercial and partnering potential.
Strategic Significance: Addressing a Large and Growing MS Market
Multiple Sclerosis represents a large global market, with approximately 2.3 million people living with MS worldwide and forecasts indicating the global MS drug market is expected to exceed USD 41 billion by 2033. Within this market, Mavenclad® (cladribine tablets) is a well-established high-efficacy therapy with annual global sales exceeding USD 1 billion and sustained double-digit growth, although systemic oral therapy can require monitoring and may be associated with adverse effects that influence real-world adoption.
Cladribine also holds promise for the treatment of additional neurodegenerative diseases, such as Myasthenia Gravis (MG).
Patent Position Strengthened - Final Granting Expected Shortly
BioNxt further confirms that patent protection for its cladribine ODF program has already been confirmed, with additional final (national level) patent grants expected shortly, strengthening the Company's intellectual property position and supporting potential future licensing and partnering opportunities.
Next Steps and Upcoming Updates
BioNxt has initiated internal screening and full evaluation of the study dataset and expects to provide additional disclosure once final calculations, interpretation, and internal validation are complete. The Company intends to share further updates regarding next development milestones in due course.
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next-generation drug delivery platforms, diagnostic screening systems, and active pharmaceutical ingredient development. Its proprietary platforms include sublingual thin films, transdermal patches, oral tablets, and a new targeted chemotherapy platform designed to deliver cancer drugs directly to tumors while reducing side effects.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient-centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co‐Founder, CEO and Director
Email: [email protected]
Phone: +1 604.250.6162
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt‐solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward‐Looking" Information
This press release contains "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian securities laws (collectively, "forward-looking information"). Forward-looking information includes, but is not limited to, statements regarding the Company's cladribine sublingual orally dissolving film ("ODF") program; the interpretation, significance, and implications of the final results received from the Company's comparative in-vivo pharmacokinetics study; the completion of the Company's internal screening, calculations, and analysis of the study dataset; the timing and content of future updates or disclosures; expectations regarding enhanced bioavailability, improved delivery efficiency, reduced systemic exposure, reduced side effects, and/or reduced required active ingredient levels; statements regarding the anticipated timing and outcome of patent granting; statements regarding the commercial relevance of comparator products; and statements regarding potential future commercialization, licensing, or partnering opportunities.
Forward-looking information is based on management's reasonable assumptions, expectations, estimates, and projections as of the date of this press release. Such statements are inherently subject to a number of risks, uncertainties, and other factors- many of which are beyond the Company's control-that may cause actual results, performance, or achievements to differ materially from those expressed or implied by such forward-looking information. These risks and uncertainties include, but are not limited to: risks related to the Company's ability to complete, validate, and interpret its internal review and calculations; the possibility that additional analysis or interpretation of the study data may yield different conclusions than those currently expected; scientific, technical, and preclinical development risks; the risk that in-vivo or animal study results may not be predictive of human outcomes; the timing, cost, conduct, and results of future studies and potential clinical trials; manufacturing, formulation, and scale-up risks; reliance on third-party contract research organizations, laboratories, and manufacturing partners; regulatory risks and the ability to obtain and maintain required approvals; intellectual property risks, including the timing, scope, validity, and enforceability of patent protection; competitive developments; and general economic, financial, and capital market conditions.
Readers are cautioned not to place undue reliance on forward-looking information. Although the Company believes that the expectations and assumptions reflected in such statements are reasonable, there can be no assurance that they will prove to be correct. Except as required by applicable securities laws, the Company undertakes no obligation to update or revise any forward-looking information, whether as a result of new information, future events, or otherwise.
Mavenclad® is a registered trademark of Merck KGaA and is not affiliated with or developed by BioNxt Solutions Inc.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
Th.Berger--AMWN

