
-
 Musetti rues 'really painful' retirement after schooling Djokovic
Musetti rues 'really painful' retirement after schooling Djokovic
-
Russian volcano puts on display in latest eruption

-
 Thailand uses contraceptive vaccine to limit wild elephant births
Thailand uses contraceptive vaccine to limit wild elephant births
-
Djokovic gets lucky to join Pegula, Rybakina in Melbourne semi-finals

-
 Trump says to 'de-escalate' Minneapolis, as aide questions agents' 'protocol'
Trump says to 'de-escalate' Minneapolis, as aide questions agents' 'protocol'
-
'Extremely lucky' Djokovic into Melbourne semi-finals as Musetti retires

-
 'Animals in a zoo': Players back Gauff call for more privacy
'Animals in a zoo': Players back Gauff call for more privacy
-
Starmer heads to China to defend 'pragmatic' partnership

-
 Uganda's Quidditch players with global dreams
Uganda's Quidditch players with global dreams
-
'Hard to survive': Kyiv's elderly shiver after Russian attacks on power and heat

-
 Polish migrants return home to a changed country
Polish migrants return home to a changed country
-
Dutch tech giant ASML posts bumper profits, eyes bright AI future

-
 South Korea's ex-first lady jailed for 20 months for corruption
South Korea's ex-first lady jailed for 20 months for corruption
-
Minnesota congresswoman unbowed after attacked with liquid

-
 Pegula must 'crack the code' in Melbourne semi-final against Rybakina
Pegula must 'crack the code' in Melbourne semi-final against Rybakina
-
Backlash as Australia kills dingoes after backpacker death

-
 Brazil declares acai a national fruit to ward off 'biopiracy'
Brazil declares acai a national fruit to ward off 'biopiracy'
-
Anisimova 'loses her mind' after Melbourne quarter-final exit

-
 Home hope Goggia on medal mission at Milan-Cortina Winter Olympics
Home hope Goggia on medal mission at Milan-Cortina Winter Olympics
-
Pegula, Rybakina to clash in Melbourne semis as Djokovic takes centre stage

-
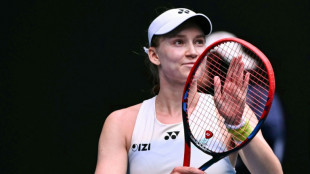 Rybakina says making Australian Open semis 'just another day'
Rybakina says making Australian Open semis 'just another day'
-
Omar attacked in Minneapolis after Trump vows to 'de-escalate'

-
 Pistons escape Nuggets rally, Thunder roll Pelicans
Pistons escape Nuggets rally, Thunder roll Pelicans
-
Dominant Pegula sets up Australian Open semi-final against Rybakina

-
 'Animals in a zoo': Swiatek backs Gauff call for more privacy
'Animals in a zoo': Swiatek backs Gauff call for more privacy
-
Japan PM's tax giveaway roils markets and worries voters

-
 Amid Ukraine war fallout, fearful Chechen women seek escape route
Amid Ukraine war fallout, fearful Chechen women seek escape route
-
Rybakina surges into Melbourne semis as Djokovic takes centre stage

-
 Dollar struggles to recover from losses after Trump comments
Dollar struggles to recover from losses after Trump comments
-
Greenland blues to Delhi red carpet: EU finds solace in India

-
 Will the EU ban social media for children in 2026?
Will the EU ban social media for children in 2026?
-
Netherlands faces 'test case' climate verdict over Caribbean island

-
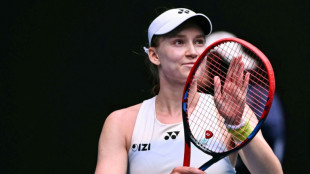 Rybakina stuns Swiatek to reach Australian Open semi-finals
Rybakina stuns Swiatek to reach Australian Open semi-finals
-
US ouster of Maduro nightmare scenario for Kim: N. Korean ex-diplomat

-
 Svitolina credits mental health break for reaching Melbourne semis
Svitolina credits mental health break for reaching Melbourne semis
-
Japan's Olympic ice icons inspire new skating generation

-
 Safe nowhere: massacre at Mexico football field sows despair
Safe nowhere: massacre at Mexico football field sows despair
-
North Korea to soon unveil 'next-stage' nuclear plans, Kim says

-
 French ex-senator found guilty of drugging lawmaker
French ex-senator found guilty of drugging lawmaker
-
US Fed set to pause rate cuts as it defies Trump pressure

-
 Sleeping with one eye open: Venezuelans reel from US strikes
Sleeping with one eye open: Venezuelans reel from US strikes
-
Venezuela's acting president says US unfreezing sanctioned funds

-
 KPop Demon Hunters star to open Women's Asian Cup
KPop Demon Hunters star to open Women's Asian Cup
-
Trump warns of 'bad things' if Republicans lose midterms

-
 Russian strikes in Ukraine kill 12, target passenger train
Russian strikes in Ukraine kill 12, target passenger train
-
With Maduro gone, Venezuelan opposition figure gets back to work

-
 Genflow Biosciences PLC Announces Administrative Approval of Grant & 2026 Priorities
Genflow Biosciences PLC Announces Administrative Approval of Grant & 2026 Priorities
-
Awesome FREE T‑Shirt Giveaway for Elektros Shareholders - Demand Is Exploding

-
 Celebrities call for action against US immigration raids
Celebrities call for action against US immigration raids
-
Rubio to warn Venezuela leader of Maduro's fate if defiant


Genflow Biosciences PLC Announces Administrative Approval of Grant & 2026 Priorities
LONDON, UK / ACCESS Newswire / January 28, 2026 / THE INFORMATION CONTAINED WITHIN THIS ANNOUNCEMENT IS DEEMED BY THE COMPANY TO CONSTITUTE INSIDE INFORMATION AS STIPULATED UNDER THE MARKET ABUSE REGULATION (EU) NO. 596/2014 AS IT FORMS PART OF UK DOMESTIC LAW PURSUANT TO THE EUROPEAN UNION (WITHDRAWAL) ACT 2018, AS AMENDED. UPON THE PUBLICATION OF THIS ANNOUNCEMENT VIA A REGULATORY INFORMATION SERVICE, THIS INFORMATION IS CONSIDERED TO BE IN THE PUBLIC DOMAIN.
Genflow Biosciences Plc
Administrative Approval of €4 Million Funding Support from Wallonia Region for GF-100; and 2026 Development Priorities
Genflow Biosciences Plc (LSE:GENF)(OTCQB:GENFF) ("Genflow" or the "Company"), a European-based biotechnology company focused on the development of gene therapies for age-related diseases, announces the administrative approval of the previously announced award of approximately €4 million in non-dilutive financial support from the Wallonia Region of Belgium, following the assenting signature of Minister Adrien Dolimont.
The funding, once received, will support the continued development of the Company's lead gene therapy candidate, GF-1002, for the treatment of Metabolic Dysfunction-Associated Steatohepatitis ("MASH"). The original RNS announcing the award can be found here.
Disbursement of the first instalment is expected no later than May 2026. The support package spans a three-year development programme and is aligned with Genflow's existing roadmap. All project-related expenses incurred during 2025 will be eligible under the terms of the programme.
Development Priorities for 2026
In 2026 Genflow's strategy emphasises pipeline discipline and capital efficiency, prioritising programmes with the strongest data, the clearest paths to value creation, and the highest potential for partner engagement. Central to this approach is the pursuit of early-stage collaboration agreements designed to secure non-dilutive funding, provide external validation of the platform, and support continued development.
With multiple anticipated data readouts, an expanding partner dialogue, and a maturing intellectual property position, Genflow believes it is well positioned to enter its next phase of development while maintaining a focus on scientific rigour, operational execution, and long-term shareholder value creation.
Dog Aging (GF-1004)
The ongoing GF-1004 dog aging study, which commenced in March 2025, is being conducted as a blinded clinical trial designed to assess potential benefits related to sarcopenia, healthspan, and lifespan-associated biomarkers.
An initial efficacy readout is expected in the first quarter of 2026. Dogs enrolled in the study are being monitored over a 180-day period, with a second efficacy assessment planned at the six-month timepoint. Results from this later evaluation are expected in June-July 2026 and are intended to assess durability and longer-term effects.
Glaucoma Programme
The Company's glaucoma programme will prioritise delivery optimisation and execution readiness. Genflow is advancing discussions with a leading lipid nanoparticle (LNP) partner to support delivery innovation and is progressing the selection of a full-service contract research organisation (CRO) to manage RNA-based formulation activities through preclinical execution.
Additional Programmes
Across the broader pipeline, Genflow continues to advance its sarcopenia programme in line with established development plans. In parallel, the ExoFastTrack initiative continues to generate data intended to support and accelerate multiple programmes across the portfolio.
Dr. Eric Leire, CEO of Genflow, commented: "The administrative approval of this funding reinforces our ability to execute against our 2026 development priorities with discipline and focus. It strengthens our capacity to advance GF-1002 while maintaining a selective, data-driven approach across the broader pipeline. As we enter the coming year, our emphasis remains on programmes with clear execution paths, strong scientific rationale, and meaningful opportunities for partnership and non-dilutive value creation."
Contacts
Genflow Biosciences | Harbor Access |
Dr Eric Leire, CEO | Jonathan Paterson, Investor Relations |
+32-477-495-881 | +1 475 477 9401 |
About Genflow Biosciences
Founded in 2020, Genflow Biosciences Plc. (LSE:GENF)(OTCQB:GENFF), a biotechnology company headquartered in the UK with R&D facilities in Belgium, is pioneering gene therapies for age-related diseases, with the goal of promoting longer and healthier lives while mitigating the financial, emotional, and social impacts of a fast-growing aging global population. Genflow's lead compound, GF-1002, works through the delivery of a centenarian variant of the SIRT6 gene which has yielded promising preclinical results. Genflow's 12-month proof-of-concept clinical trial evaluating their SIRT6-centenarian gene therapy in aged dogs began in March 2025. Other programs, include a clinical trial that will explore the potential benefits of GF-1002 in treating MASH (Metabolic Dysfunction-Associated Steatohepatitis), the most prevalent chronic liver disease for which there is no effective treatments. Please visit www.genflowbio.com and follow the Company on LinkedIn and X.
DISCLAIMER
The contents of this announcement have been prepared by, and are the sole responsibility of, the Company.
This announcement may contain forward-looking statements. The forward-looking statements include, but are not limited to, statements regarding the Company's or the Directors' expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statement that refers to projections, forecasts or other characterisations of future events or circumstances, including any underlying assumptions, is a forward-looking statement. The words "anticipate", "believe", "continue", "could", "estimate", "expect", "intend", "may", "might", "plan", "possible", "potential", "predict", "project", "seek", "should", "would" and similar expressions, or in each case their negatives, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
Forward-looking statements include all matters that are not historical facts. Forward-looking statements are based on the current expectations and assumptions regarding the Company, the business, the economy and other future conditions. Because forward-looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Forward-looking statements are not guarantees of future performance and the Company's actual financial condition, actual results of operations and financial performance, and the development of the industries in which it operates or will operate, may differ materially from those made in or suggested by the forward-looking statements contained in this announcement. In addition, even if the Company's financial condition, results of operations and the development of the industries in which it operates or will operate, are consistent with the forward-looking statements contained in this announcement, those results or developments may not be indicative of financial condition, results of operations or developments in subsequent periods. Important factors that could cause actual results to differ materially from those in the forward-looking statements include regional, national or global, political, economic, social, business, technological, competitive, market and regulatory conditions.
Any forward-looking statement contained in this announcement applies only as of the date of this announcement and is expressly qualified in its entirety by these cautionary statements. Factors or events that could cause the Company's actual plans or results to differ may emerge from time to time, and it is not possible for the Company to predict all of them. The Company expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained in this announcement to reflect any change in its expectations or any change in events, conditions or circumstances on which any forward-looking statement contained in this announcement is based, unless required to do so by applicable law, the Prospectus Regulation Rules, the Listing Rules, the Disclosure Guidance and Transparency Rules of the FCA or the UK Market Abuse Regulation.
This information is provided by RNS, the news service of the London Stock Exchange. RNS is approved by the Financial Conduct Authority to act as a Primary Information Provider in the United Kingdom. Terms and conditions relating to the use and distribution of this information may apply. For further information, please contact [email protected] or visit www.rns.com.
SOURCE: Genflow Biosciences PLC
View the original press release on ACCESS Newswire
B.Finley--AMWN