
-
 Minneapolis activists track Trump's immigration enforcers
Minneapolis activists track Trump's immigration enforcers
-
Court orders Dutch to protect Caribbean island from climate change

-
 Sterling agrees Chelsea exit after troubled spell
Sterling agrees Chelsea exit after troubled spell
-
Rules-based trade with US is 'over': Canada central bank head

-
 Lucas Paqueta signs for Flamengo in record South American deal
Lucas Paqueta signs for Flamengo in record South American deal
-
Holocaust survivor urges German MPs to tackle resurgent antisemitism

-
 'Extraordinary' trove of ancient species found in China quarry
'Extraordinary' trove of ancient species found in China quarry
-
Villa's Tielemans ruled out for up to 10 weeks

-
 Google unveils AI tool probing mysteries of human genome
Google unveils AI tool probing mysteries of human genome
-
UK proposes to let websites refuse Google AI search

-
 'I wanted to die': survivors recount Mozambique flood terror
'I wanted to die': survivors recount Mozambique flood terror
-
Trump says 'time running out' as Iran threatens tough response

-
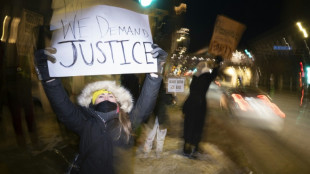 Trump issues fierce warning to Minneapolis mayor over immigration
Trump issues fierce warning to Minneapolis mayor over immigration
-
Anglican church's first female leader confirmed at London service

-
 Germany cuts growth forecast as recovery slower than hoped
Germany cuts growth forecast as recovery slower than hoped
-
Amazon to cut 16,000 jobs worldwide

-
 One dead, five injured in clashes between Colombia football fans
One dead, five injured in clashes between Colombia football fans
-
Greenland dispute is 'wake-up call' for Europe: Macron

-
 Dollar halts descent, gold keeps climbing before Fed update
Dollar halts descent, gold keeps climbing before Fed update
-
US YouTuber IShowSpeed gains Ghanaian nationality at end of Africa tour

-
 Sweden plans to ban mobile phones in schools
Sweden plans to ban mobile phones in schools
-
Turkey football club faces probe over braids clip backing Syrian Kurds

-
 Deutsche Bank offices searched in money laundering probe
Deutsche Bank offices searched in money laundering probe
-
US embassy angers Danish veterans by removing flags

-
 Netherlands 'insufficiently' protects Caribbean island from climate change: court
Netherlands 'insufficiently' protects Caribbean island from climate change: court
-
Fury confirms April comeback fight against Makhmudov

-
 Susan Sarandon to be honoured at Spain's top film awards
Susan Sarandon to be honoured at Spain's top film awards
-
Trump says 'time running out' as Iran rejects talks amid 'threats'

-
 Spain eyes full service on train tragedy line in 10 days
Spain eyes full service on train tragedy line in 10 days
-
Greenland dispute 'strategic wake-up call for all of Europe,' says Macron

-
 'Intimidation and coercion': Iran pressuring families of killed protesters
'Intimidation and coercion': Iran pressuring families of killed protesters
-
'Pride of the entire nation': Israel holds funeral for last Gaza hostage

-
 Europe urged to 'step up' on defence as Trump upends ties
Europe urged to 'step up' on defence as Trump upends ties
-
Aki a doubt for Ireland's Six Nations opener over disciplinary issue

-
 West Ham sign Fulham winger Traore
West Ham sign Fulham winger Traore
-
Relentless Sinner sets up Australian Open blockbuster with Djokovic

-
 Israel prepares to bury last Gaza hostage
Israel prepares to bury last Gaza hostage
-
Iran rejects talks with US amid military 'threats'

-
 Heart attack ends iconic French prop Atonio's career
Heart attack ends iconic French prop Atonio's career
-
SKorean chip giant SK hynix posts record operating profit for 2025

-
 Greenland's elite dogsled unit patrols desolate, icy Arctic
Greenland's elite dogsled unit patrols desolate, icy Arctic
-
'Extremely lucky' Djokovic says he must improve after Australian Open scare

-
 Starmer arrives in China to defend 'pragmatic' partnership
Starmer arrives in China to defend 'pragmatic' partnership
-
Musetti rues 'really painful' retirement after schooling Djokovic

-
 Russian volcano puts on display in latest eruption
Russian volcano puts on display in latest eruption
-
Thailand uses contraceptive vaccine to limit wild elephant births

-
 Djokovic gets lucky to join Pegula, Rybakina in Melbourne semi-finals
Djokovic gets lucky to join Pegula, Rybakina in Melbourne semi-finals
-
Trump says to 'de-escalate' Minneapolis, as aide questions agents' 'protocol'

-
 'Extremely lucky' Djokovic into Melbourne semi-finals as Musetti retires
'Extremely lucky' Djokovic into Melbourne semi-finals as Musetti retires
-
'Animals in a zoo': Players back Gauff call for more privacy


Ensysce Biosciences Provides Enrollment Update on Pivotal Phase 3 Trial of PF614, Its Next-Generation Opioid for Severe Acute Pain
~ Engineered to Deliver Potent Pain Relief with Built-In Abuse Protection ~
SAN DIEGO, CA / ACCESS Newswire / January 28, 2026 / Ensysce Biosciences, Inc. (NASDAQ:ENSC) ("Ensysce" or the "Company"), a clinical-stage pharmaceutical company pioneering novel solutions for severe pain with built-in abuse and overdose protection, today announced it has enrolled 50% of subjects targeted for interim review in its pivotal Phase 3 clinical trial of PF614, the Company's next-generation opioid candidate engineered to deliver powerful pain relief with built-in abuse protection..
Enrollment began in late December 2025 and is progressing rapidly across three U.S. clinical sites: CenExel JBR (Salt Lake City, Utah); CenExel Atlanta (Decatur, Georgia); and ERG-HD Research, LLC (Houston, Texas). The study is being led by Dr. Todd Bertoch, Dr. Jessica McCoun, and Dr. D'Aunno, recognized experts in anesthesiology and pain management.
The pivotal PF614-301 trial is a multicenter, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of PF614 for the treatment of moderate to severe pain following abdominoplasty. The study is designed to demonstrate PF614's ability to deliver consistent, clinically meaningful post-surgical pain relief using twice-daily dosing.
PF614 leverages Ensysce's proprietary chemical activation technology, which is designed to keep the opioid inactive until swallowed, limiting the impact of tampering and dose manipulation while enabling extended-release pain control. This approach is intended to address one of the central challenges in modern pain care: delivering opioid-level efficacy while reducing the risks of abuse and misuse.
"This milestone reflects the strength of our execution to provide better options to treat severe acute pain," said Dr. Lynn Kirkpatrick, Chief Executive Officer of Ensysce. "Patients recovering from major surgery still require opioid level analgesia for effective pain control. PF614 is designed to deliver that level of relief reliably and predictably while incorporating intrinsic safeguards that are absent from conventional opioids. Achieving this enrollment milestone in the early stages of this Phase 3 program brings us meaningfully closer to delivering interim data and advancing what we believe could be a new standard in acute pain management. We are pleased to share this encouraging study update."
About Ensysce Biosciences
Ensysce Biosciences is a clinical-stage pharmaceutical company disrupting the pain treatment landscape with its proprietary Trypsin-Activated Abuse Protection (TAAP™) and Multi-Pill Abuse Resistance (MPAR®) platforms. By engineering opioids with intrinsic safeguards against tampering, misuse, and overdose, Ensysce aims to offer safer, life-saving options for patients in need of powerful pain relief. Learn more at: www.ensysce.com
Forward-Looking Statements
Statements contained in this press release that are not purely historical may be deemed to be forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. Without limiting the foregoing, the use of words such as "may," "intends," "can," "might," "will," "expect," "plan," "possible," "believe" and other similar expressions are intended to identify forward-looking statements. The product candidates discussed are in clinic and not approved and there can be no assurance that the clinical programs will be successful in demonstrating safety and/or efficacy, that Ensysce will not encounter problems or delays in clinical development, or that any product candidate will ever receive regulatory approval or be successfully commercialized. All forward-looking statements are based on estimates and assumptions by Ensysce's management that, although Ensysce believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Ensysce expected. In addition, Ensysce's business is subject to additional risks and uncertainties, including among others, the initiation and conduct of preclinical studies and clinical trials; the timing and availability of data from preclinical studies and clinical trials; expectations for regulatory submissions and approvals; potential safety concerns related to, or efficacy of, Ensysce's product candidates; the availability or commercial potential of product candidates; the ability of Ensysce to fund its continued operations, including its planned clinical trials; the dilutive effect of stock issuances from our fundraising; and Ensysce's and its partners' ability to perform under their license, collaboration and manufacturing arrangements. These statements are also subject to a number of material risks and uncertainties that are described in Ensysce's most recent quarterly report on Form 10-Q and current reports on Form 8-K, which are available, free of charge, at the SEC's website at www.sec.gov. Any forward-looking statement speaks only as of the date on which it was made. Ensysce undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required under applicable law.
Ensysce Biosciences Company Contact:
Lynn Kirkpatrick, Ph.D.
Chief Executive Officer
(858) 263-4196
Ensysce Biosciences Investor Relations Contact:
Shannon Devine
MZ North America
Main: 203-741-8811
[email protected]
SOURCE: Ensysce Biosciences
View the original press release on ACCESS Newswire
F.Dubois--AMWN
