
-
 Minneapolis activists track Trump's immigration enforcers
Minneapolis activists track Trump's immigration enforcers
-
Court orders Dutch to protect Caribbean island from climate change

-
 Sterling agrees Chelsea exit after troubled spell
Sterling agrees Chelsea exit after troubled spell
-
Rules-based trade with US is 'over': Canada central bank head

-
 Lucas Paqueta signs for Flamengo in record South American deal
Lucas Paqueta signs for Flamengo in record South American deal
-
Holocaust survivor urges German MPs to tackle resurgent antisemitism

-
 'Extraordinary' trove of ancient species found in China quarry
'Extraordinary' trove of ancient species found in China quarry
-
Villa's Tielemans ruled out for up to 10 weeks

-
 Google unveils AI tool probing mysteries of human genome
Google unveils AI tool probing mysteries of human genome
-
UK proposes to let websites refuse Google AI search

-
 'I wanted to die': survivors recount Mozambique flood terror
'I wanted to die': survivors recount Mozambique flood terror
-
Trump says 'time running out' as Iran threatens tough response

-
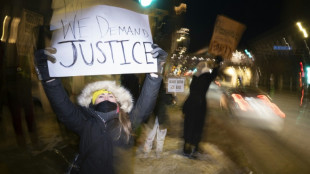 Trump issues fierce warning to Minneapolis mayor over immigration
Trump issues fierce warning to Minneapolis mayor over immigration
-
Anglican church's first female leader confirmed at London service

-
 Germany cuts growth forecast as recovery slower than hoped
Germany cuts growth forecast as recovery slower than hoped
-
Amazon to cut 16,000 jobs worldwide

-
 One dead, five injured in clashes between Colombia football fans
One dead, five injured in clashes between Colombia football fans
-
Greenland dispute is 'wake-up call' for Europe: Macron

-
 Dollar halts descent, gold keeps climbing before Fed update
Dollar halts descent, gold keeps climbing before Fed update
-
US YouTuber IShowSpeed gains Ghanaian nationality at end of Africa tour

-
 Sweden plans to ban mobile phones in schools
Sweden plans to ban mobile phones in schools
-
Turkey football club faces probe over braids clip backing Syrian Kurds

-
 Deutsche Bank offices searched in money laundering probe
Deutsche Bank offices searched in money laundering probe
-
US embassy angers Danish veterans by removing flags

-
 Netherlands 'insufficiently' protects Caribbean island from climate change: court
Netherlands 'insufficiently' protects Caribbean island from climate change: court
-
Fury confirms April comeback fight against Makhmudov

-
 Susan Sarandon to be honoured at Spain's top film awards
Susan Sarandon to be honoured at Spain's top film awards
-
Trump says 'time running out' as Iran rejects talks amid 'threats'

-
 Spain eyes full service on train tragedy line in 10 days
Spain eyes full service on train tragedy line in 10 days
-
Greenland dispute 'strategic wake-up call for all of Europe,' says Macron

-
 'Intimidation and coercion': Iran pressuring families of killed protesters
'Intimidation and coercion': Iran pressuring families of killed protesters
-
'Pride of the entire nation': Israel holds funeral for last Gaza hostage

-
 Europe urged to 'step up' on defence as Trump upends ties
Europe urged to 'step up' on defence as Trump upends ties
-
Aki a doubt for Ireland's Six Nations opener over disciplinary issue

-
 West Ham sign Fulham winger Traore
West Ham sign Fulham winger Traore
-
Relentless Sinner sets up Australian Open blockbuster with Djokovic

-
 Israel prepares to bury last Gaza hostage
Israel prepares to bury last Gaza hostage
-
Iran rejects talks with US amid military 'threats'

-
 Heart attack ends iconic French prop Atonio's career
Heart attack ends iconic French prop Atonio's career
-
SKorean chip giant SK hynix posts record operating profit for 2025

-
 Greenland's elite dogsled unit patrols desolate, icy Arctic
Greenland's elite dogsled unit patrols desolate, icy Arctic
-
'Extremely lucky' Djokovic says he must improve after Australian Open scare

-
 Starmer arrives in China to defend 'pragmatic' partnership
Starmer arrives in China to defend 'pragmatic' partnership
-
Musetti rues 'really painful' retirement after schooling Djokovic

-
 Russian volcano puts on display in latest eruption
Russian volcano puts on display in latest eruption
-
Thailand uses contraceptive vaccine to limit wild elephant births

-
 Djokovic gets lucky to join Pegula, Rybakina in Melbourne semi-finals
Djokovic gets lucky to join Pegula, Rybakina in Melbourne semi-finals
-
Trump says to 'de-escalate' Minneapolis, as aide questions agents' 'protocol'

-
 'Extremely lucky' Djokovic into Melbourne semi-finals as Musetti retires
'Extremely lucky' Djokovic into Melbourne semi-finals as Musetti retires
-
'Animals in a zoo': Players back Gauff call for more privacy


Tenon(R) Medical Issues Shareholder Letter and Provides Preliminary Revenue for the Fourth Quarter and Full Year 2025
Fourth Quarter 2025 Revenue of $1.45 to $1.48 Million, representing growth of ~90% year over year.
Full Year 2025 Revenue of $3.91 to $3.94 Million, representing growth of ~20% year over year.
LOS GATOS, CA / ACCESS Newswire / January 28, 2026 / Tenon Medical, Inc. (NASDAQ:TNON) ("Tenon" or the "Company"), a company redefining care for patients suffering from sacro-pelvic disorders, today issued a letter to shareholders from Steve Foster, President and Chief Executive Officer.
Dear Fellow Shareholders,
2025 was marked by strong commercial execution as we advanced innovation and expanded our differentiated solutions in the marketplace. We delivered both sequential and year over year revenue growth and strengthened our liquidity position, placing Tenon on firmer financial footing heading into 2026. Our expanding product portfolio-driven by the acquisition of sacroiliac joint-specific assets from SiVantage and the recent FDA clearance and commercialization of the SImmetry®+ SI Joint Fusion System-positions us to scale revenue and address a broader range of sacro-pelvic fixation and fusion needs. Entering 2026, we have laid a strong foundation for sustained growth through portfolio diversification, commercial expansion, and improved operational alignment.
Preliminary Fourth Quarter & Full Year 2025 Revenue
Following record revenue growth in the third quarter, the Company is reporting preliminary, unaudited fourth quarter revenue of approximately $1.45 to $1.48 million for the period ended December 31, 2025, representing approximately 90% growth compared to the fourth quarter ended December 31, 2024. Preliminary unaudited full year 2025 revenue is expected to be approximately $3.91 to $3.94 million, representing approximately 20% year over year growth. Revenue acceleration in the second half of 2025 was driven by strong procedural volume momentum, which we expect to continue into 2026. This momentum was primarily fueled by adoption among new physician users of both the Catamaran® and SImmetry+ systems.
These revenue results are preliminary and subject to adjustment upon completion of customary year-end audit procedures. The Company expects to report fourth-quarter and full-year 2025 financial results in March 2026.
Major Company Milestones in 2025
Throughout 2025, our primary focus was advancing product innovation and generating robust clinical outcomes to transform Tenon into a multi-product, multi-approach company. Today, we offer physicians differentiated technologies that support customized treatment strategies for patients with sacro-pelvic disorders, utilizing novel, true fusion solutions backed by compelling clinical evidence. Key milestones in 2025 include:
Transformational Acquisition of the SiVantage® SImmetry / SImmetry+ Technologies
The acquisition of the SiVantage portfolio added active clinical cases, revenue-generating technologies, and a robust development pipeline to Tenon. This transaction strengthened our commercial organization by adding proven commercialization professionals and expanding the breadth of our product offering. These enhancements will support sustainable top-line growth. The SImmetry and SImmetry+ sacroiliac joint fusion systems bring a well-established clinical foundation and a differentiated fusion approach that complements our existing Catamaran platform.
Catamaran® SE SI Joint Fusion System Full Commercial Launch
In 2025, we completed the full commercial launch of the Catamaran SE SI Joint Fusion System, further strengthening our competitive position in the sacroiliac joint fusion market. Catamaran SE features a reduced implant profile, providing physicians greater flexibility when treating patients with smaller SI joint anatomy or performing revision procedures. The system maintains a minimally invasive inferior-posterior approach and incorporates a proprietary instrument set designed to support procedural efficiency.
MAINSAIL™ Clinical Momentum from Second Peer-Reviewed Study Publication
Clinical momentum continued with the publication of a second peer-reviewed 12-month study from the MAINSAIL trial, reinforcing our "fixation until fusion" strategy. The study demonstrated strong patient outcomes, including meaningful improvements in pain and disability scores, as well as 12-month CT-confirmed fusion outcomes. Notably, 83% of patients demonstrated unequivocal evidence of fusion with bridging bone across the SI joint at 12 months. The study also reported no serious adverse events, re-operations, or reinterventions, and 83% of patients reported high satisfaction with their outcomes.
Matthew Davies, MD, a board-certified neurosurgeon at Orthopaedic Associates of Duluth in Duluth, MN, and principal investigator will present the 12-month outcomes data from the Mainsail Study during the scientific session in late February at this year's AANS/CNS Spine Summit Meeting in Phoenix, Arizona.
FDA Clearance for Expanded Catamaran Indication in Augmenting Thoracolumbar Fusion
In the first quarter of 2025, Tenon received FDA clearance for an expanded indication for the Catamaran SI Joint Fusion System to be used in augmenting thoracolumbar fusion. The sacral pelvic component of these complex spine procedures is an area of increased focus where the need for innovative solutions is evident. With this clearance, Catamaran may be utilized to augment spinal fusion procedures providing immobilization and stabilization of the SI joint, offering additional support at the base of lumbar or thoracolumbar fusion constructs.
2026 Outlook
We believe 2026 will be a milestone year for Tenon, supported by a multi-platform sacro-pelvic fusion strategy that meaningfully strengthens our competitive position and enables physicians to tailor treatment to individual patient anatomy and pathology. This differentiated approach enhances our ability to drive adoption, increase procedure volumes, and capture a greater share of a rapidly expanding market, supporting long-term value creation for our shareholders.
Looking ahead, we remain focused on accelerating adoption across our expanded product portfolio and leveraging recent regulatory and clinical milestones to support continued commercial growth. With a strong foundation in place, we are confident in our ability to scale operations, deepen market penetration, and deliver sustained value to our shareholders in the years ahead.
Sincerely,
Steve Foster
President and Chief Executive Officer
About Tenon Medical, Inc.
Tenon Medical, Inc., a medical device company formed in 2012, has developed The Catamaran SI Joint Fusion System that offers a novel, less invasive approach to the SI joint using a single, robust titanium implant. The system features the Catamaran Fixation Device which passes through both the axial and sagittal planes of the ilium and sacrum, stabilizing and transfixing the SI Joint along its longitudinal axis. The angle and trajectory of the Catamaran surgical approach is also designed to provide a pathway away from critical neural and vascular structures and into the strongest cortical bone. Since the national launch of the Catamaran SI Joint Fusion System in October 2022, Tenon is focused on three commercial opportunities with its System in the SI Joint market which include: 1) Primary SI Joint procedures, 2) Revision procedures of failed SI Joint implants and 3) Augmenting spinal fusion. For more information, please visit www.tenonmed.com.
The Tenon Medical logo shown above, and Catamaran®, PiSIF®, CAT PiSIF®, ETAD®, Posterior Inferior Sacroiliac Fusion®, CAT SIJ Fusion System®, Catamaran SIJ Fusion System®, Catamaran Inferior Posterior Fusion System®, Catamaran Transfixation Fusion System®, Catamaran Transfixation Fusion Device®, SImmetry® are registered trademarks of Tenon Medical, Inc. MAINSAILTM, and SImmetry+ are also trademarks of Tenon Medical, Inc.
Safe Harbor
This press release contains "forward-looking statements," which are statements related to events, results, activities, or developments that Tenon expects, believes, or anticipates will or may occur in the future. Forward-looking often contains words such as "intends," "estimates," "anticipates," "hopes," "projects," "plans," "expects," "seek," "believes," "see," "should," "will," "would," "target," "aims," and similar expressions and the negative versions thereof. Such statements are based on Tenon's experience and perception of current conditions, trends, expected future developments and other factors it believes are appropriate under the circumstances, and speak only as of the date made. Forward-looking statements are inherently uncertain and actual results may differ materially from assumptions, estimates or expectations reflected or contained in the forward-looking statements as a result of various factors. For details on the uncertainties that may cause our actual results to be materially different than those expressed in our forward-looking statements, please review our Annual Report on 10-K on file with the Securities and Exchange Commission at www.sec.gov, particularly the information contained in the section entitled "Risk Factors". We undertake no obligation to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise unless required by law.
Investor Contact
Shannon Devine
MZ North America
203-741-8811
[email protected]
SOURCE: Tenon Medical, Inc.
View the original press release on ACCESS Newswire
Y.Kobayashi--AMWN
