
-
 Australia's Head backs struggling opening partner Weatherald
Australia's Head backs struggling opening partner Weatherald
-
'Make emitters responsible': Thailand's clean air activists

-
 Zelensky looks to close out Ukraine peace deal at Trump meet
Zelensky looks to close out Ukraine peace deal at Trump meet
-
MCG curator in 'state of shock' after Ashes Test carnage

-
 Texans edge Chargers to reach NFL playoffs
Texans edge Chargers to reach NFL playoffs
-
Osimhen and Mane score as Nigeria win to qualify, Senegal draw

-
 Osimhen stars as Nigeria survive Tunisia rally to reach second round
Osimhen stars as Nigeria survive Tunisia rally to reach second round
-
How Myanmar's junta-run vote works, and why it might not

-
 Watkins wants to sicken Arsenal-supporting family
Watkins wants to sicken Arsenal-supporting family
-
Arsenal hold off surging Man City, Villa as Wirtz ends drought

-
 Late penalty miss denies Uganda AFCON win against Tanzania
Late penalty miss denies Uganda AFCON win against Tanzania
-
Watkins stretches Villa's winning streak at Chelsea

-
 Zelensky stops in Canada en route to US as Russia pummels Ukraine
Zelensky stops in Canada en route to US as Russia pummels Ukraine
-
Arteta salutes injury-hit Arsenal's survival spirit

-
 Wirtz scores first Liverpool goal as Anfield remembers Jota
Wirtz scores first Liverpool goal as Anfield remembers Jota
-
Mane rescues AFCON draw for Senegal against DR Congo

-
 Arsenal hold off surging Man City, Wirtz breaks Liverpool duck
Arsenal hold off surging Man City, Wirtz breaks Liverpool duck
-
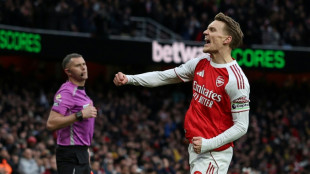
Arsenal ignore injury woes to retain top spot with win over Brighton
-
 Sealed with a kiss: Guardiola revels in Cherki starring role
Sealed with a kiss: Guardiola revels in Cherki starring role
-
UK launches paid military gap-year scheme amid recruitment struggles

-
 Jota's children join tributes as Liverpool, Wolves pay respects
Jota's children join tributes as Liverpool, Wolves pay respects
-
'Tired' Inoue beats Picasso by unanimous decision to end gruelling year

-
 Thailand and Cambodia declare truce after weeks of clashes
Thailand and Cambodia declare truce after weeks of clashes
-
Netanyahu to meet Trump in US on Monday

-
 US strikes targeted IS militants, Lakurawa jihadists, Nigeria says
US strikes targeted IS militants, Lakurawa jihadists, Nigeria says
-
Cherki stars in Man City win at Forest

-
 Schwarz records maiden super-G success, Odermatt fourth
Schwarz records maiden super-G success, Odermatt fourth
-
Russia pummels Kyiv ahead of Zelensky's US visit

-
 Smith laments lack of runs after first Ashes home Test loss for 15 years
Smith laments lack of runs after first Ashes home Test loss for 15 years
-
Russian barrage on Kyiv kills one, leaves hundreds of thousands without power

-
 Stokes, Smith agree two-day Tests not a good look after MCG carnage
Stokes, Smith agree two-day Tests not a good look after MCG carnage
-
Stokes hails under-fire England's courage in 'really special' Test win

-
 What they said as England win 4th Ashes Test - reaction
What they said as England win 4th Ashes Test - reaction
-
Hong Kongers bid farewell to 'king of umbrellas'

-
 England snap 15-year losing streak to win chaotic 4th Ashes Test
England snap 15-year losing streak to win chaotic 4th Ashes Test
-
Thailand and Cambodia agree to 'immediate' ceasefire

-
 Closing 10-0 run lifts Bulls over 76ers while Pistons fall
Closing 10-0 run lifts Bulls over 76ers while Pistons fall
-
England 77-2 at tea, need 98 more to win chaotic 4th Ashes Test

-
 Somalia, African nations denounce Israeli recognition of Somaliland
Somalia, African nations denounce Israeli recognition of Somaliland
-
England need 175 to win chaotic 4th Ashes Test

-
 Cricket Australia boss says short Tests 'bad for business' after MCG carnage
Cricket Australia boss says short Tests 'bad for business' after MCG carnage
-
Russia lashes out at Zelensky ahead of new Trump talks on Ukraine plan

-
 Six Australia wickets fall as England fight back in 4th Ashes Test
Six Australia wickets fall as England fight back in 4th Ashes Test
-
New to The Street Show #710 Airs Tonight at 6:30 PM EST on Bloomberg Television

-
 Dental Implant Financing and Insurance Options in Georgetown, TX
Dental Implant Financing and Insurance Options in Georgetown, TX
-
Man Utd made to 'suffer' for Newcastle win, says Amorim

-
 Morocco made to wait for Cup of Nations knockout place after Egypt advance
Morocco made to wait for Cup of Nations knockout place after Egypt advance
-
Key NFL week has playoff spots, byes and seeds at stake

-
 Morocco forced to wait for AFCON knockout place after Mali draw
Morocco forced to wait for AFCON knockout place after Mali draw
-
Dorgu delivers winner for depleted Man Utd against Newcastle


Mixed results for Moderna mRNA flu vaccine trial
US biotech company Moderna said Thursday it had mixed results from a large-scale trial of its mRNA flu shot, based on the same technology used in its successful Covid-19 vaccine.
Moderna's experimental mRNA-1010 flu shot is "quadrivalent," meaning it targets four strains of flu: A/H1N1, A/H3N2, B/Yamagata and B/Victoria -- selected based on recommendations by the World Health Organization (WHO).
The Massachusetts-based company said that its flu shot generated an immune response against influenza A strains that was equal or superior to that of already licensed vaccines.
However, it fell short of the already approved vaccines against strains of the less-common influenza B, Moderna said in a statement.
"Today's results represent an important step forward in the development of mRNA-based influenza vaccines," Moderna president Stephen Hoge said.
"We have already updated the vaccine that we believe could improve immune responses against influenza B and will seek to quickly confirm those improvements in an upcoming clinical study."
The Phase 3 trial of the mRNA shot involved 6,102 adults in Argentina, Australia, Colombia, Panama, and the Philippines during the Southern Hemisphere influenza season.
Participants received a single dose of mRNA-1010 or a single dose of a licensed influenza vaccine.
Moderna said 70 percent of the mRNA-1010 recipients reported adverse reactions such as headaches, swelling and fatigue compared to 48 percent in the other group.
Virus strains have to be selected six to nine months before the vaccines are intended to be used, and their efficacy is approximately 40 to 60 percent.
Moderna is simultaneously conducting an efficacy trial of its vaccine.
Moderna and other vaccine manufacturers, including Sanofi, hope that mRNA technology -- which provokes an immune response by delivering genetic molecules containing the code for key parts of a pathogen into human cells -- can accelerate immunization development and production, and heighten efficacy.
There are some three to five million severe influenza cases annually worldwide and between 290,000 and 650,000 deaths, the WHO says.
Moderna's stock price was down more than six percent in after-hours trading in New York.
L.Durand--AMWN



