-
 Women sommeliers are cracking male-dominated wine world open
Women sommeliers are cracking male-dominated wine world open
-
Exhibition of Franco-Chinese print master Zao Wou-Ki opens in Hong Kong

-
 Myanmar junta denies killing civilians in hospital strike
Myanmar junta denies killing civilians in hospital strike
-
Why SpaceX IPO plan is generating so much buzz

-
 Thailand continues Cambodia strikes despite Trump truce calls
Thailand continues Cambodia strikes despite Trump truce calls
-
US envoy to meet Zelensky, Europe leaders in Berlin this weekend

-
 North Korea acknowledges its troops cleared mines for Russia
North Korea acknowledges its troops cleared mines for Russia
-
US unseals warrant for tanker seized off Venezuelan coast

-
 Cambodia says Thailand still bombing hours after Trump truce call
Cambodia says Thailand still bombing hours after Trump truce call
-
Machado urges pressure so Maduro understands 'he has to go'

-
 Best Gold Investment Companies in USA Announced (Augusta Precious Metals, Lear Capital, Robinhood IRA and More Ranked)
Best Gold Investment Companies in USA Announced (Augusta Precious Metals, Lear Capital, Robinhood IRA and More Ranked)
-
Leinster stutter before beating Leicester in Champions Cup

-
 World stocks mostly slide, consolidating Fed-fuelled gains
World stocks mostly slide, consolidating Fed-fuelled gains
-
Crypto firm Tether bids for Juventus, is quickly rebuffed

-
 Union sink second-placed Leipzig to climb in Bundesliga
Union sink second-placed Leipzig to climb in Bundesliga
-
US Treasury lifts sanctions on Brazil Supreme Court justice

-
 UK king shares 'good news' that cancer treatment will be reduced in 2026
UK king shares 'good news' that cancer treatment will be reduced in 2026
-
Wembanyama expected to return for Spurs in NBA Cup clash with Thunder

-
 Five takeaways from Luigi Mangione evidence hearings
Five takeaways from Luigi Mangione evidence hearings
-
UK's king shares 'good news' that cancer treatment will be reduced in 2026

-
 Steelers' Watt undergoes surgery to repair collapsed lung
Steelers' Watt undergoes surgery to repair collapsed lung
-
Iran detains Nobel-prize winner in 'brutal' arrest

-
 NBA Cup goes from 'outside the box' idea to smash hit
NBA Cup goes from 'outside the box' idea to smash hit
-
UK health service battles 'super flu' outbreak

-
 Can Venezuela survive US targeting its oil tankers?
Can Venezuela survive US targeting its oil tankers?
-
Democrats release new cache of Epstein photos

-
 Colombia's ELN guerrillas place communities in lockdown citing Trump 'intervention' threats
Colombia's ELN guerrillas place communities in lockdown citing Trump 'intervention' threats
-
'Don't use them': Tanning beds triple skin cancer risk, study finds
-
 Nancy aims to restore Celtic faith with Scottish League Cup final win
Nancy aims to restore Celtic faith with Scottish League Cup final win
-
Argentina fly-half Albornoz signs for Toulon until 2030

-
 Trump says Thailand, Cambodia have agreed to stop border clashes
Trump says Thailand, Cambodia have agreed to stop border clashes
-
Salah in Liverpool squad for Brighton after Slot talks - reports

-
 Marseille coach tips Greenwood as 'potential Ballon d'Or'
Marseille coach tips Greenwood as 'potential Ballon d'Or'
-
Draw marks 'starting gun' toward 2026 World Cup, Vancouver says

-
 Thai PM says asked Trump to press Cambodia on border truce
Thai PM says asked Trump to press Cambodia on border truce
-
Salah admired from afar in his Egypt home village as club tensions swirl

-
 World stocks retrench, consolidating Fed-fuelled gains
World stocks retrench, consolidating Fed-fuelled gains
-
Brazil left calls protests over bid to cut Bolsonaro jail time

-
 Trump attack on Europe migration 'disaster' masks toughening policies
Trump attack on Europe migration 'disaster' masks toughening policies
-
US plan sees Ukraine joining EU in 2027, official tells AFP

-
 'Chilling effect': Israel reforms raise press freedom fears
'Chilling effect': Israel reforms raise press freedom fears
-
Iran frees child bride sentenced to death over husband's killing: activists

-
 No doubting Man City boss Guardiola's passion says Toure
No doubting Man City boss Guardiola's passion says Toure
-
Youthful La Rochelle name teen captain for Champions Cup match in South Africa

-
 World stocks consolidate Fed-fuelled gains
World stocks consolidate Fed-fuelled gains
-
British 'Aga saga' author Joanna Trollope dies aged 82

-
 Man Utd sweat on Africa Cup of Nations trio
Man Utd sweat on Africa Cup of Nations trio
-
EU agrees three-euro small parcel tax to tackle China flood

-
 Taylor Swift breaks down in Eras documentary over Southport attack
Taylor Swift breaks down in Eras documentary over Southport attack
-
Maresca 'relaxed' about Chelsea's rough patch


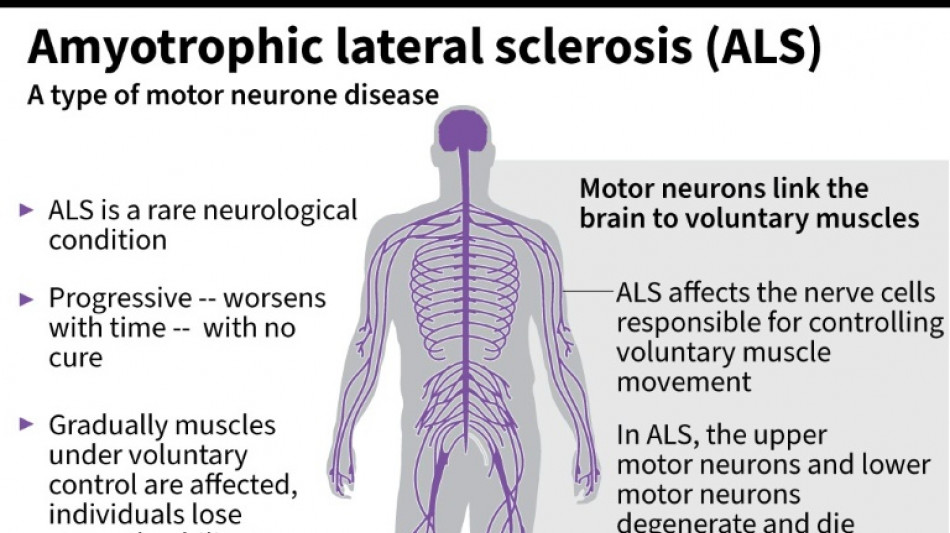
Every month counts: European ALS patients want new drugs
Olivier Goy is running out of time.
The French entrepreneur was diagnosed in 2020 with amyotrophic lateral sclerosis (ALS) -- the incurable neurodegenerative disease that normally claims the lives of patients within three to five years.
There are new treatments that have given patients hope of being able to extend their lives by an invaluable few months, but the approval process in Europe is taking time, infuriating desperate patients.
"When you are certain to die soon, patients and some doctors are ready to take some risks," Goy told AFP.
In response to the lack of new treatments in his native France, the founder of the fintech start-up October spends 3,000 euros ($3,180) every month to buy the ingredients to make his own drugs.
ALS, also known as Lou Gehrig's disease, attacks the motor nerve cells in the brain and spinal cord, progressively paralysing muscles until patients cannot walk, eat, speak or breathe.
Around one in 10,000 people have the disease in the EU, according to the European Medicines Agency.
The drug Riluzole, which has been available in Europe and the UK since the 1990s, is capable of prolonging the lives of patients by around three months.
But otherwise, no new treatment has been approved in Europe for more than two decades.
- 'First hope in 20 years' -
A new treatment called AMX0035 was given the green light in the United States and Canada last year.
"It is the first hope we have had in 20 years: the first drug which is aimed at everyone and which had results" suggesting up to six months in added life expectancy, said Sabine Turgeman, head of the French Association for Research into ALS.
But the extent of the benefits of AMX0035 remains unclear. The US Food and Drug Administration approved the drug, sold under the name Relyvrio, based on the results of a single Phase 2 trial that involved just 137 participants.
The drug's developer, Amylyx Pharmaceuticals, is conducting larger, more comprehensive trials, with results expected in 2024.
Amylyx said earlier this month that the European Union's drug watchdog EMA is reviewing its submission for approval and it expects a decision in the first half of this year.
But for those with the disease, every delay represents a significant amount of the time they have left.
"It's not going fast enough," Turgeman said. "This disease is not on bureaucratic time".
For European patients who cannot afford to import their own ingredients like Goy, the only way to get access to new treatments is to join a clinical trial.
But such trials have very specific criteria for selection -- and even if a patient gets in, there is a chance they will be in the group given a placebo.
- 'Totally abandoned' -
Given how swiftly the disease progresses, patients and families are pressing for more options.
"We feel totally abandoned," said Sophie Garofalo, whose brother was diagnosed with ALS five years ago.
His family tried to enter him into clinical trials, "but either he does not meet the criteria, or the trials have already started," she said.
"He is ready to take anything, try everything".
French pharmaceutical company AB Science is developing another potential treatment using the drug masitinib, which initial results suggest could add months to the lives of patients.
The firm's CEO Alain Moussy said that because "time is very limited" for ALS patients, there should be more flexibility in the approval system.
"What degree of risk should be taken? That's for the health agencies to answer -- but they can guided by policymakers and patients," he said.
O.M.Souza--AMWN

