
-
 George backs England to 'kick on' after Six Nations rout of Wales
George backs England to 'kick on' after Six Nations rout of Wales
-
Malinin upstaged as Japan keep pressure on USA in skating team event

-
 Vail's golden comets Vonn and Shiffrin inspire those who follow
Vail's golden comets Vonn and Shiffrin inspire those who follow
-
Veteran French politician loses culture post over Epstein links

-
 Japan's Kimura wins Olympic snowboard big air gold
Japan's Kimura wins Olympic snowboard big air gold
-
Arteta backs confident Gyokeres to hit 'highest level'

-
 Hojlund the hero as Napoli snatch late win at Genoa
Hojlund the hero as Napoli snatch late win at Genoa
-
England's Arundell 'frustrated' despite hat-trick in Wales romp

-
 Lollobrigida skates to first Italian gold of Winter Olympics on her birthday
Lollobrigida skates to first Italian gold of Winter Olympics on her birthday
-
Arundell hat-trick inspires England thrashing of Wales in Six Nations opener

-
 Chile's climate summit chief to lead plastic pollution treaty talks
Chile's climate summit chief to lead plastic pollution treaty talks
-
Rosenior hails 'unstoppable' Palmer after treble tames Wolves

-
 French ex-minister offers resignation from Paris cultural hub over Epstein links
French ex-minister offers resignation from Paris cultural hub over Epstein links
-
New NBA dunk contest champ assured and shooting stars return

-
 Shiffrin says will use lessons learnt from Beijing flop at 2026 Games
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games
-
Takaichi tipped for big win as Japan votes

-
 Lens return top of Ligue 1 with win over Rennes
Lens return top of Ligue 1 with win over Rennes
-
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow

-
 Demonstrators in Berlin call for fall of Iran's Islamic republic
Demonstrators in Berlin call for fall of Iran's Islamic republic
-
'Free the mountains!": clashes at Milan protest over Winter Olympics

-
 Townsend accepts pressure will mount on him after Italy defeat
Townsend accepts pressure will mount on him after Italy defeat
-
BMW iX3 new style and design

-
 Suryakumar's 84 leads India to opening win over USA in T20 World Cup
Suryakumar's 84 leads India to opening win over USA in T20 World Cup
-
Lollobrigida skates to first Italian gold of Milan-Cortina Games

-
 Barca beat Mallorca to extend Liga lead
Barca beat Mallorca to extend Liga lead
-
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank

-
 Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga
-
'Free the mountains!": protest in Milan over Winter Olympics
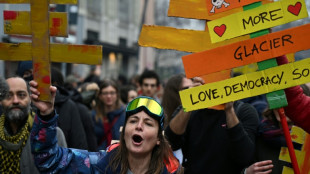
-
 Gyokeres double helps Arsenal stretch Premier League lead
Gyokeres double helps Arsenal stretch Premier League lead
-
New Skoda Epiq: modern with range

-
 Six Nations misery for Townsend as Italy beat sorry Scotland
Six Nations misery for Townsend as Italy beat sorry Scotland
-
Spain, Portugal face fresh storms, torrential rain

-
 Opinions of Zuckerberg hang over social media addiction trial jury selection
Opinions of Zuckerberg hang over social media addiction trial jury selection
-
Over 2,200 IS detainees transferred to Iraq from Syria: Iraqi official

-
 Norway's Ruud tops Olympic men's freeski slopestyle qualifying
Norway's Ruud tops Olympic men's freeski slopestyle qualifying
-
Czech qualifier Bejlek claims first title in Abu Dhabi

-
 French duo reach Shanghai, completing year-and-a-half walk
French duo reach Shanghai, completing year-and-a-half walk
-
Australian snowboarder James eyes elusive Olympic gold

-
 Sequins and snow: Eva Adamczykova makes Olympic return
Sequins and snow: Eva Adamczykova makes Olympic return
-
Vonn set for Olympic medal bid after successful downhill training

-
 Shepherd takes hat-trick as West Indies beat Scotland in T20 World Cup
Shepherd takes hat-trick as West Indies beat Scotland in T20 World Cup
-
Sausages will sell after thrill-seeker Von Allmen wins Olympic downhill

-
 Swiss racer Von Allmen wins first gold of Winter Olympics
Swiss racer Von Allmen wins first gold of Winter Olympics
-
'Wake up': Mum sparks comeback after scare for freeski star Gu

-
 Von Allmen wins men's Olympic downhill gold, first of Games
Von Allmen wins men's Olympic downhill gold, first of Games
-
First medals up for grabs at Winter Olympics

-
 Afghanistan captain Khan harbours dream of playing in Kabul
Afghanistan captain Khan harbours dream of playing in Kabul
-
Lindsey Vonn completes second Winter Olympics downhill training run

-
 Freeski star Gu survives major scare in Olympic slopestyle
Freeski star Gu survives major scare in Olympic slopestyle
-
Iran FM looks to more nuclear talks, but warns US


Moderna says infant Covid vaccine succeeded in trial
US biotech firm Moderna on Wednesday said it was pursuing regulatory approval for its Covid vaccine in children aged six months to six years after the two-shot regimen was found to be safe and produced a strong immune response.
Specifically, two doses of 25 micrograms given to this age group generated similar levels of antibodies to two doses of 100 micrograms given to young people aged 18-25, indicating there would be similar levels of protection.
Based on the data, Moderna said it would submit authorization requests to the US Food and Drug Administration (FDA), European Medicines Agency (EMA) and other global regulators in the coming weeks.
The results "are good news for parents of children under six years of age," said CEO Stephane Bancel in a statement.
"We now have clinical data on the performance of our vaccine from infants six months of age through older adults."
The company did however find relatively low vaccine efficacy against infection, with its trial taking place during the Omicron wave.
Vaccine efficacy in children six months to two years was 43.7 percent, and vaccine efficacy was 37.5 percent in the two to under six years age group.
Moderna said this was consistent with what had been observed among adults and the company was evaluating a third dose to lift efficacy levels.
The trial comprised 11,700 pediatric volunteers in the United States and Canada, including 4,200 aged two to six years and 2,500 aged six to two months.
The company added that, after consulting with the FDA, it is also applying to be authorized among children six to 11 for two-doses of 50 micrograms, and updating its application for authorization in kids aged 12 through 17.
The EMA and other regulators have already authorized the Moderna vaccine in these age groups.
Last month, the FDA postponed a meeting of a panel to consider the Pfizer-BioNTech Covid vaccine for children younger than five, saying it required additional data on third doses. The companies said they expected that data to be ready by April.
D.Cunningha--AMWN


