-
 Naib's fifty lifts Afghanistan to 182-6 against New Zealand
Naib's fifty lifts Afghanistan to 182-6 against New Zealand
-
Paul Thomas Anderson wins top director prize for 'One Battle After Another'

-
 De Beers sale drags in diamond doldrums
De Beers sale drags in diamond doldrums
-
NFL embraces fashion as league seeks new audiences

-
 What's at stake for Indian agriculture in Trump's trade deal?
What's at stake for Indian agriculture in Trump's trade deal?
-
Real Madrid can wait - Siraj's dream night after late T20 call-up

-
 Castle's monster night fuels Spurs, Rockets rally to beat Thunder
Castle's monster night fuels Spurs, Rockets rally to beat Thunder
-
Japan votes in snow-hit snap polls as Takaichi eyes strong mandate

-
 Pakistan's capital picks concrete over trees, angering residents
Pakistan's capital picks concrete over trees, angering residents
-
Berlin's crumbling 'Russian houses' trapped in bureaucratic limbo

-
 Neglected killer: kala-azar disease surges in Kenya
Neglected killer: kala-azar disease surges in Kenya
-
Super Bowl set for Patriots-Seahawks showdown as politics swirl

-
 Sengun shines as Rockets rally to beat NBA champion Thunder
Sengun shines as Rockets rally to beat NBA champion Thunder
-
Matsuyama grabs PGA Phoenix Open lead with Hisatsune one back

-
 Washington Post CEO out after sweeping job cuts
Washington Post CEO out after sweeping job cuts
-
Haiti's transitional council hands power to PM

-
 N. Korea to hold party congress in February, first since 2021
N. Korea to hold party congress in February, first since 2021
-
Thailand votes after three leaders in two years

-
 Swiss joy as Von Allmen wins first gold of Winter Olympics
Swiss joy as Von Allmen wins first gold of Winter Olympics
-
George backs England to 'kick on' after Six Nations rout of Wales

-
 Malinin upstaged as Japan keep pressure on USA in skating team event
Malinin upstaged as Japan keep pressure on USA in skating team event
-
Vail's golden comets Vonn and Shiffrin inspire those who follow

-
 Veteran French politician loses culture post over Epstein links
Veteran French politician loses culture post over Epstein links
-
Japan's Kimura wins Olympic snowboard big air gold

-
 Arteta backs confident Gyokeres to hit 'highest level'
Arteta backs confident Gyokeres to hit 'highest level'
-
Hojlund the hero as Napoli snatch late win at Genoa

-
 England's Arundell 'frustrated' despite hat-trick in Wales romp
England's Arundell 'frustrated' despite hat-trick in Wales romp
-
Lollobrigida skates to first Italian gold of Winter Olympics on her birthday

-
 Arundell hat-trick inspires England thrashing of Wales in Six Nations opener
Arundell hat-trick inspires England thrashing of Wales in Six Nations opener
-
Chile's climate summit chief to lead plastic pollution treaty talks

-
 Rosenior hails 'unstoppable' Palmer after treble tames Wolves
Rosenior hails 'unstoppable' Palmer after treble tames Wolves
-
French ex-minister offers resignation from Paris cultural hub over Epstein links

-
 New NBA dunk contest champ assured and shooting stars return
New NBA dunk contest champ assured and shooting stars return
-
Shiffrin says will use lessons learnt from Beijing flop at 2026 Games

-
 Takaichi tipped for big win as Japan votes
Takaichi tipped for big win as Japan votes
-
Lens return top of Ligue 1 with win over Rennes

-
 Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
Shiffrin learning from Beijing lessons ahead of Milan-Cortina bow
-
Demonstrators in Berlin call for fall of Iran's Islamic republic

-
 'Free the mountains!": clashes at Milan protest over Winter Olympics
'Free the mountains!": clashes at Milan protest over Winter Olympics
-
Townsend accepts pressure will mount on him after Italy defeat

-
 BMW iX3 new style and design
BMW iX3 new style and design
-
Suryakumar's 84 leads India to opening win over USA in T20 World Cup

-
 Lollobrigida skates to first Italian gold of Milan-Cortina Games
Lollobrigida skates to first Italian gold of Milan-Cortina Games
-
Barca beat Mallorca to extend Liga lead

-
 Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
Gyokeres lifts Arsenal nine clear as Man Utd pile pressure on Frank
-
Late Guirassy winner for Dortmund trims Bayern's lead atop Bundesliga

-
 'Free the mountains!": protest in Milan over Winter Olympics
'Free the mountains!": protest in Milan over Winter Olympics
-
Gyokeres double helps Arsenal stretch Premier League lead

-
 New Skoda Epiq: modern with range
New Skoda Epiq: modern with range
-
Six Nations misery for Townsend as Italy beat sorry Scotland


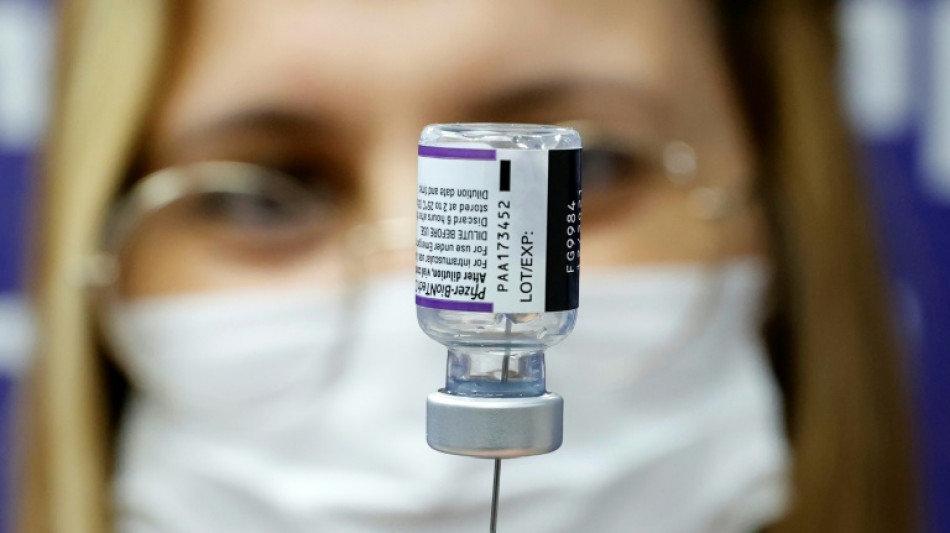
Pfizer Covid vaccine for under-fives effective with three doses
The Pfizer/BioNTech Covid vaccine is safe and effective for children aged six months to under five years when given in three doses, the companies said in a statement Monday.
The announcement comes as the US Food and Drug Administration (FDA) is planning meetings in the coming weeks to weigh authorizing Covid vaccines among the youngest children, the only age group who are not yet eligible in most countries, a source of concern to many parents.
Pfizer/BioNTech evaluated three doses, given at three micrograms, in a clinical trial and found the vaccine evoked a strong immune response. Side effects were similar in the vaccine and placebo groups.
Vaccine efficacy was 80.3 percent, according to a preliminary estimate.
"We are pleased that our formulation for the youngest children, which we carefully selected to be one-tenth of the dose strength for adults, was well tolerated and produced a strong immune response," said Pfizer CEO Albert Bourla in a statement.
"We look forward to soon completing our submissions to regulators globally with the hope of making this vaccine available to younger children as quickly as possible, subject to regulatory authorization," he added.
The FDA has tentatively scheduled three dates in June where experts will meet and likely decide whether to authorize the Pfizer Covid vaccine for under-fives and the Moderna vaccine for under-sixes, which is given as two shots of 25 micrograms.
The agency was originally set to evaluate the Pfizer vaccine given as two doses in February, but data showed it did not provoke a strong enough immune response in children aged two to four. The FDA then asked to see data for a third shot.
- Data welcomed -
According to the new data, 1,678 children received a third dose at least two months after the second dose, at a time when Omicron was the predominant variant.
An analysis of a subset of participants showed antibody levels were similar to 16- to 25-year-olds who were given the full strength vaccine at two doses.
No new adverse events were identified, and the majority of side effects were mild or moderate.
"Three doses of (Pfizer's) Covid vaccine appear to be very safe and highly effective in preventing not only severe disease, hospitalization, and death from Covid, but even symptomatic Covid at a time when Omicron was the dominant variant," Celine Gounder, editor-at-large for public health at Kaiser Health News told AFP.
"However, we know that protection against SARS-CoV-2 infection and milder symptomatic disease wanes over time," added Gounder, an infectious disease specialist and epidemiologist.
"Pfizer is reporting follow-up data only out to seven days after the third dose of vaccine. It's too early to say how the three-dose series would perform out to several months or a year."
Jeremy Faust, of Brigham and Women's Hospital Department of Emergency Medicine, told AFP: "My first impression is very positive. These numbers are exactly the kinds of signals we wanted to see."
"I wish the two-dose series had worked for Pfizer/BioNTech. It didn't. But the three-dose series appears to have given these very young children the protection we want them to have," the doctor added.
If and when both Pfizer's and Moderna's vaccines are authorized, US parents will have to consider whether they want their children to receive Moderna's two dose vaccine -- which will offer faster protection -- or Pfizer's three doses -- which will take longer to be effective but may ultimately be more protective.
Pfizer's announcement comes one week after US health authorities gave the green light for the company's Covid booster shots to be administered to children age five to 11.
Severe disease from Covid is very rare among under-fives but can occur, with 477 US deaths in this age group since the start of the pandemic, or about 0.1 percent of all deaths.
Children can also contract a rare post-viral condition called multisystem inflammatory syndrome in children (MIS-C), which has affected some 8,210 US children and killed 68.
Like adults, some children who get Covid may go on to develop long Covid, with new, ongoing or returning symptoms, including brain fog and fatigue.
J.Oliveira--AMWN


