
-
 Pirovano doubles up with second Val di Fassa downhill win
Pirovano doubles up with second Val di Fassa downhill win
-
Rapper-turned-politician Shah unseats former Nepal PM in own constituency

-
 Beating Italy is not a 'God-given right', says Wales coach Tandy
Beating Italy is not a 'God-given right', says Wales coach Tandy
-
Sri Lanka to treat Iranian sailors according to 'international law'

-
 New Zealand want to 'break a few hearts' in World Cup final
New Zealand want to 'break a few hearts' in World Cup final
-
Farrell welcomes bonus-point win over 'tough' Welsh

-
 Iran vows no surrender as air strikes hit Tehran airport
Iran vows no surrender as air strikes hit Tehran airport
-
Hamilton says 'not where we wanted or expected' for Australian GP

-
 Pole-sitter Russell says his Mercedes more go-kart than 'bouncing bus'
Pole-sitter Russell says his Mercedes more go-kart than 'bouncing bus'
-
Google gives CEO new pay deal worth up to $692 million

-
 Thousands of Taiwan fans turn Tokyo blue at World Baseball Classic
Thousands of Taiwan fans turn Tokyo blue at World Baseball Classic
-
Verstappen baffled by crash in Australian Grand Prix qualifying

-
 Russell leads Mercedes 1-2 for Australian GP as Verstappen crashes
Russell leads Mercedes 1-2 for Australian GP as Verstappen crashes
-
'Grateful' Osaka returns to action with Indian Wells win

-
 Israel fires 'broad-scale' strikes on Tehran as war hits 2nd week
Israel fires 'broad-scale' strikes on Tehran as war hits 2nd week
-
Rapper-turned-politician looks set for landslide Nepal election win

-
 Russian strike on Kharkiv apartment block kills three
Russian strike on Kharkiv apartment block kills three
-
Judge homers as USA cruise past Brazil in World Baseball Classic

-
 Russian strike on Kharkiv appartment block kills three
Russian strike on Kharkiv appartment block kills three
-
Grabbing the bull by the tail: Venezuela's cowboy sport

-
 Russell tops final practice in Melbourne as Antonelli crashes heavily
Russell tops final practice in Melbourne as Antonelli crashes heavily
-
Vibes war? Trump pitches Iran conflict on 'feeling'

-
 Nepal's rapper-turned-politician looks set for landslide win
Nepal's rapper-turned-politician looks set for landslide win
-
Tatum's 'emotional' return sparks Celtics over Mavs

-
 Rising US fuel prices risk sparking domestic wildfire for Trump
Rising US fuel prices risk sparking domestic wildfire for Trump
-
Questions over AI capability as tech guides Iran strikes

-
 Trump convenes Latin American leaders to curb crime, immigration
Trump convenes Latin American leaders to curb crime, immigration
-
Dipylon Medical Expands Access to Clinical Microbiology Equipment for Modern Diagnostic Laboratories

-
 Bestday Safaris Launches Affordable Tanzania Safari Tours for International Travelers
Bestday Safaris Launches Affordable Tanzania Safari Tours for International Travelers
-
All Home Care Matters Takes Over Leadership Role of AlzAuthors

-
 Venezuela inflation hit 475% in 2025, the world's highest level
Venezuela inflation hit 475% in 2025, the world's highest level
-
Only Iran's 'unconditional surrender' can end war: Trump

-
 Former 100m champion Kerley banned two years over whereabouts failures
Former 100m champion Kerley banned two years over whereabouts failures
-
Sabalenka opens Indian Wells bid with dominant win

-
 Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh
Doris relieved Ireland's slim title hopes intact after 'scrappy' win over Welsh
-
Man City aren't a 'complete team' admits Guardiola

-
 Arteta warns Arsenal to preserve reputation in Mansfield clash
Arteta warns Arsenal to preserve reputation in Mansfield clash
-
Timothee Chalamet taken to task over opera, ballet dig

-
 Ireland keep title hopes alive in thrilling win over Wales
Ireland keep title hopes alive in thrilling win over Wales
-
Hungary has not returned cash seized from bank workers, Kyiv says

-
 Napoli secure first Serie A home win since January
Napoli secure first Serie A home win since January
-
Valverde strikes late as Real Madrid beat Celta Vigo

-
 PSG beaten by Monaco ahead of Chelsea Champions League showdown
PSG beaten by Monaco ahead of Chelsea Champions League showdown
-
Liverpool tame Wolves to reach FA Cup quarter-finals

-
 Kane-less Bayern brush aside Gladbach to continue title march
Kane-less Bayern brush aside Gladbach to continue title march
-
Only nine commercial ships detected crossing Hormuz Strait since Monday
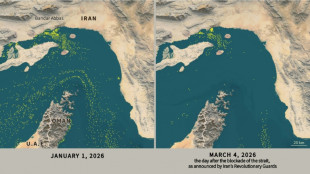
-
 Berger extends lead midway through Arnold Palmer Invitational
Berger extends lead midway through Arnold Palmer Invitational
-
Paralympics open with Russian athletes booed in ceremony

-
 Cuba 'next' on agenda, after Iran: Trump
Cuba 'next' on agenda, after Iran: Trump
-
Zverev leads way into Indian Wells third round


enVVeno Medical Highlights Successful 2024 VEITH Symposium with Launch of Recap Website
Interviews with participating patients from the VenoValve® U.S. Pivotal Trial
Webcast replay from with the presenting Primary Investigators
Presentation with data from the VenoValve U.S. Pivotal Trial
Access the Recap Website Here!
enVVeno Medical Corporation (NASDAQ:NVNO) ("enVVeno" or the "Company"), a company setting new standards of care for the treatment of venous disease, today announced the launch of a recap website which highlights the Company's participation at the recently held 51st Annual Vascular and Endovascular, Techniques and Horizons (VEITH) Symposium. Materials accessible on the recap website include interviews with two patients and two Principal Investigators who participated in the VenoValve U.S. Pivotal Trial as well as data presented at the symposium.

"While our data is compelling, we think it is equally important to hear the stories behind the data from both physicians and patients who have first-hand experience with the VenoValve," said Robert Berman, enVVeno Medical's Chief Executive Officer. "It is physicians and patients that will ultimately drive our commercial success following FDA approval. This is the second group of patients and third group of primary investigators whose perspectives we have made available. It takes an organic network of physician and patient advocates for a new device to succeed and anyone who takes the time to engage with these materials will recognize the strong foundation we've established."
Access the enVVeno Medical VEITH Symposium Recap Site at envveno.com/veith-2024
Severe, deep venous Chronic Venous Insufficiency (CVI) is a debilitating disease that is most often caused by blood clots (deep vein thromboses or DVTs) in the deep veins of the leg. When valves inside of the veins of the leg fail, blood flows in the wrong direction and pools in the lower leg, causing pressure within the veins of the leg to increase (venous hypertension). Symptoms of severe CVI include leg swelling, pain, edema, and in the most severe cases, recurrent open sores known as venous ulcers. The disease can severely impact everyday functions such as sleeping, bathing, dressing, and walking, and is known to result in high rates of depression and anxiety. There are currently no effective treatments for severe CVI of the deep vein system caused by valvular incompetence. Estimates indicate that CVI costs the U.S. healthcare system in excess of $4 billion each year.
The VenoValve® is a potential first-in-class, surgical replacement venous valve for patients with severe deep venous CVI. The Company estimates that there are approximately 2.5 million potential new patients each year in the U.S. that could be candidates for the VenoValve. The Company is also developing enVVe®, a next-generation, transcatheter based replacement venous valve, that could appeal to an even larger market in terms of both patients and physicians.
About enVVeno Medical Corporation
enVVeno Medical (NASDAQ:NVNO) is an Irvine, California-based, late clinical-stage medical device Company focused on the advancement of innovative bioprosthetic (tissue-based) solutions to improve the standard of care for the treatment of venous disease. The Company's lead product, the VenoValve®, is a first-in-class surgical replacement venous valve being developed for the treatment of deep venous Chronic Venous Insufficiency (CVI). The Company is also developing a non-surgical, transcatheter based replacement venous valve for the treatment of deep venous CVI called enVVe®. CVI occurs when valves inside of the veins of the leg become damaged, resulting in the backwards flow of blood (reflux), blood pooling in the lower leg, increased pressure in the veins of the leg (venous hypertension) and in severe cases, venous ulcers that are difficult to heal and become chronic. Both the VenoValve and enVVe are designed to act as one-way valves, to help assist in propelling blood up the leg, and back to the heart and lungs. The VenoValve is currently being evaluated in the SAVVE U.S. pivotal study and the Company is currently performing the final testing necessary to seek approval for the pivotal trial for enVVe.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of enVVeno Medical Corporation (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results and timing (may differ significantly from those set forth or implied in the forward-looking statements. Forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
###
INVESTOR CONTACT:
Jenene Thomas, JTC Team, LLC
[email protected]
(908) 824-0775
SOURCE: enVVeno Medical Corporation
O.M.Souza--AMWN


