-
 Redknapp's Gold Cup dream sparked by late grandmother
Redknapp's Gold Cup dream sparked by late grandmother
-
Trump tries to reset presidency in State of the Union speech

-
 Harden hails 'special' Cavs after emphatic win over Knicks
Harden hails 'special' Cavs after emphatic win over Knicks
-
Division, theater and one golden moment as Trump addresses Congress

-
 Humble Japan ready to win hearts at Women's Asian Cup
Humble Japan ready to win hearts at Women's Asian Cup
-
New Zealand mayor swims to allay sewage contamination fears

-
 Trump vows 'turnaround for the ages' in State of the Union
Trump vows 'turnaround for the ages' in State of the Union
-
Marquez targets eighth MotoGP title as season opens in Thailand

-
 Months after floods, Indonesian survivors frustrated by slow response
Months after floods, Indonesian survivors frustrated by slow response
-
Tech firms lead Asian markets rally as Seoul, Tokyo hit records

-
 Nepali migrant workers influence polls, but can't vote
Nepali migrant workers influence polls, but can't vote
-
Canadians are choosing when to die, often with a smile

-
 Trump to promise 'turnaround for the ages' in State of the Union
Trump to promise 'turnaround for the ages' in State of the Union
-
Economy not Russia is big fear on Finland's closed frontier

-
 Alexandria bids farewell to historic tram in latest urban upheaval
Alexandria bids farewell to historic tram in latest urban upheaval
-
The veteran 'insider' shaping Iran's nuclear policy

-
 'Jaws' harpoon gun and 'Star Wars' treasures lead LA film and TV auction
'Jaws' harpoon gun and 'Star Wars' treasures lead LA film and TV auction
-
Brazil prosecutor urges politicians' conviction in murder of black councilwoman

-
 Starved of fuel, Cubans scramble to make ends meet
Starved of fuel, Cubans scramble to make ends meet
-
Giant killers Bodo/Glimt continue remarkable rise with Inter triumph

-
 Bodo/Glimt knock Inter out of Champions League as Newcastle, Atletico reach last 16
Bodo/Glimt knock Inter out of Champions League as Newcastle, Atletico reach last 16
-
Australian Open chief Tiley steps down to take top US job

-
 Crime capital no more: El Salvador tourism boosted by Bukele
Crime capital no more: El Salvador tourism boosted by Bukele
-
FIFA boss 'very reassured' about World Cup in Mexico despite violence

-
 15 states sue Trump administration over child vaccine policy
15 states sue Trump administration over child vaccine policy
-
Rescuers search for missing after deluge kills 30 in Brazil

-
 Newcastle complete cruise into Champions League last 16
Newcastle complete cruise into Champions League last 16
-
Leverkusen through to Champions League last 16 after Olympiacos draw

-
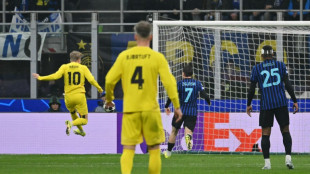 Bodo/Glimt sink Inter to continue Champions League fairy tale
Bodo/Glimt sink Inter to continue Champions League fairy tale
-
Tech shares rebound as markets weigh AI impacts
-
 Puerto Vallarta: the Mexican paradise in flames over the killing of 'El Mencho'
Puerto Vallarta: the Mexican paradise in flames over the killing of 'El Mencho'
-
Sorloth treble helps Atletico past Brugge into Champions League last 16

-
 Louvre president hands in resignation to Macron: Elysee
Louvre president hands in resignation to Macron: Elysee
-
Iran says deal 'within reach' ahead of US talks

-
 Torrential rains leave 25 dead in Brazil, dozens missing
Torrential rains leave 25 dead in Brazil, dozens missing
-
Northeast US faces power cuts and school closures after snowstorm

-
 US abstains in UN vote voicing support for Ukraine
US abstains in UN vote voicing support for Ukraine
-
Lebanon fears Israeli strikes if Iran situation escalates

-
 Trump seeks to strike back in crucial State of the Union
Trump seeks to strike back in crucial State of the Union
-
World-class Brook played 'the best innings of his life' - Afridi

-
 US appeals WTO ruling in dispute by China over clean energy subsidies
US appeals WTO ruling in dispute by China over clean energy subsidies
-
Guadalajara: World Cup host city rocked by narco violence

-
 Briiliant Brook 100 puts England into T20 World Cup semi-finals
Briiliant Brook 100 puts England into T20 World Cup semi-finals
-
Germany's Merz heads to China for talks centred on trade

-
 Briiliant Brook 100 puts England into T20 World Cups semi-finals
Briiliant Brook 100 puts England into T20 World Cups semi-finals
-
Warner Bros. 'reviewing' new takeover bid from Paramount

-
 US told EU it 'stands' by tariff deal: trade chief
US told EU it 'stands' by tariff deal: trade chief
-
Torrential rains leave 23 dead in Brazil, dozens missing

-
 UK govt says will release files on 'rude' ex-prince Andrew
UK govt says will release files on 'rude' ex-prince Andrew
-
Nearly an own gull! CPR performed on bird at Turkey football match


IGC Pharma Reports 50% Patient Enrollment Milestone in Phase 2 CALMA Alzheimer's Agitation Trial
POTOMAC, MD / ACCESS Newswire / September 22, 2025 / IGC Pharma, Inc. (NYSE American:IGC), a clinical-stage pharmaceutical company focused on therapies for Alzheimer's disease, today announced it has reached a key enrollment milestone of 50% for its ongoing Phase 2 CALMA clinical trial evaluating IGC-AD1 for the treatment of agitation in Alzheimer's disease.
This milestone marks a significant step in advancing IGC-AD1, the Company's proprietary formulation that combines low concentrations of delta-9 tetrahydrocannabinol (THC) a naturally derived cannabinoid, with another active pharmaceutical ingredient. Preclinical and clinical evidence suggest that IGC-AD1 has a multi-modal mechanism of action, reducing agitation while also demonstrating potential disease-modifying effects through activity against beta-amyloid plaques, tau tangles and diminished mitochondrial functioning.
"CALMA is a multicenter, double blind, placebo controlled, randomized trial. Enrolling 50% of the target is a key achievement for the CALMA program," said Ram Mukunda, CEO of IGC Pharma. "Agitation affects up to 76% of Alzheimer's patients and remains one of the most disruptive and underserved symptoms, with current treatments carrying black box warnings or serious side effects. IGC-AD1 offers the potential for a safer, better tolerated therapy that could meaningfully improve quality of life for both patients and caregivers. With encouraging data, from our Phase 1 and from the Phase 2 interim analysis, showing reductions in agitation, we are building strong momentum toward full enrollment and remain on track with our goal of completing the trial in early 2026."
In 2025, new sites were added to broaden geographic reach and enhance patient diversity, including BayCare Health System (Florida), Hamilton Health Sciences (Ontario), Butler Hospital's Memory and Aging Program (Rhode Island), Lynn Health Science Institute (Oklahoma), Island Health's Royal Jubilee Hospital (British Columbia), and SCB Research (Puerto Rico). With enrollment accelerating across this network of leading centers, IGC Pharma remains on track to complete recruitment in calendar 2026.
To learn more about IGC Pharma and the CALMA trial, please visit www.igcpharma.com.
About IGC Pharma (dba IGC):
IGC Pharma (NYSE American: IGC) is a clinical-stage biotechnology company leveraging AI to develop innovative treatments for Alzheimer's and metabolic disorders. Our lead asset, IGC-AD1, is a cannabinoid-based therapy currently in a Phase 2 trial (CALMA) for agitation in Alzheimer's dementia. Our pipeline includes TGR-63, targeting amyloid plaques, and early-stage programs focused on neurodegeneration, tau proteins, and metabolic dysfunctions. We integrate AI to accelerate drug discovery, optimize clinical trials, and enhance patient targeting. With 30 patent filings and a commitment to innovation, IGC Pharma is advancing breakthrough therapies.
Forward-Looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K and it quarterly report on Form 10-Q filed with the SEC on June 27, 2025, and August 14, 2025, respectively as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur. IGC Pharma, Inc. assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
Contact Information
Rosalyn Christian / John Nesbett
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
View the original press release on ACCESS Newswire
Ch.Havering--AMWN