-
 Trump to promise 'turnaround for the ages' in State of the Union
Trump to promise 'turnaround for the ages' in State of the Union
-
Economy not Russia is big fear on Finland's closed frontier

-
 Alexandria bids farewell to historic tram in latest urban upheaval
Alexandria bids farewell to historic tram in latest urban upheaval
-
The veteran 'insider' shaping Iran's nuclear policy

-
 'Jaws' harpoon gun and 'Star Wars' treasures lead LA film and TV auction
'Jaws' harpoon gun and 'Star Wars' treasures lead LA film and TV auction
-
Brazil prosecutor urges politicians' conviction in murder of black councilwoman

-
 Starved of fuel, Cubans scramble to make ends meet
Starved of fuel, Cubans scramble to make ends meet
-
Giant killers Bodo/Glimt continue remarkable rise with Inter triumph

-
 Bodo/Glimt knock Inter out of Champions League as Newcastle, Atletico reach last 16
Bodo/Glimt knock Inter out of Champions League as Newcastle, Atletico reach last 16
-
Australian Open chief Tiley steps down to take top US job

-
 Crime capital no more: El Salvador tourism boosted by Bukele
Crime capital no more: El Salvador tourism boosted by Bukele
-
FIFA boss 'very reassured' about World Cup in Mexico despite violence

-
 15 states sue Trump administration over child vaccine policy
15 states sue Trump administration over child vaccine policy
-
Rescuers search for missing after deluge kills 30 in Brazil

-
 Newcastle complete cruise into Champions League last 16
Newcastle complete cruise into Champions League last 16
-
Leverkusen through to Champions League last 16 after Olympiacos draw

-
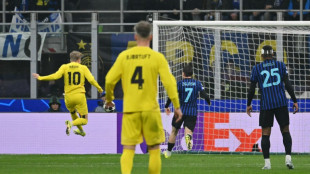 Bodo/Glimt sink Inter to continue Champions League fairy tale
Bodo/Glimt sink Inter to continue Champions League fairy tale
-
Tech shares rebound as markets weigh AI impacts
-
 Puerto Vallarta: the Mexican paradise in flames over the killing of 'El Mencho'
Puerto Vallarta: the Mexican paradise in flames over the killing of 'El Mencho'
-
Sorloth treble helps Atletico past Brugge into Champions League last 16

-
 Louvre president hands in resignation to Macron: Elysee
Louvre president hands in resignation to Macron: Elysee
-
Iran says deal 'within reach' ahead of US talks

-
 Torrential rains leave 25 dead in Brazil, dozens missing
Torrential rains leave 25 dead in Brazil, dozens missing
-
Northeast US faces power cuts and school closures after snowstorm

-
 US abstains in UN vote voicing support for Ukraine
US abstains in UN vote voicing support for Ukraine
-
Lebanon fears Israeli strikes if Iran situation escalates

-
 Trump seeks to strike back in crucial State of the Union
Trump seeks to strike back in crucial State of the Union
-
World-class Brook played 'the best innings of his life' - Afridi

-
 US appeals WTO ruling in dispute by China over clean energy subsidies
US appeals WTO ruling in dispute by China over clean energy subsidies
-
Guadalajara: World Cup host city rocked by narco violence

-
 Briiliant Brook 100 puts England into T20 World Cup semi-finals
Briiliant Brook 100 puts England into T20 World Cup semi-finals
-
Germany's Merz heads to China for talks centred on trade

-
 Briiliant Brook 100 puts England into T20 World Cups semi-finals
Briiliant Brook 100 puts England into T20 World Cups semi-finals
-
Warner Bros. 'reviewing' new takeover bid from Paramount

-
 US told EU it 'stands' by tariff deal: trade chief
US told EU it 'stands' by tariff deal: trade chief
-
Torrential rains leave 23 dead in Brazil, dozens missing

-
 UK govt says will release files on 'rude' ex-prince Andrew
UK govt says will release files on 'rude' ex-prince Andrew
-
Nearly an own gull! CPR performed on bird at Turkey football match

-
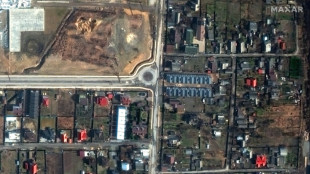 How AFP has used data analysis to cover the Ukraine war
How AFP has used data analysis to cover the Ukraine war
-
Paris says US envoy pledges not to 'interfere' in France affairs

-
 Iran says students must respect 'red lines' after protests
Iran says students must respect 'red lines' after protests
-
Italian biathlete Giacomel has heart surgery after Olympic withdrawal

-
 Gazans salvage ancient books in mosque library damaged by war
Gazans salvage ancient books in mosque library damaged by war
-
Farhan scores 63 as England restrict Pakistan to 164-9

-
 Stocks bounce as traders assess AI fallout, tariffs
Stocks bounce as traders assess AI fallout, tariffs
-
Brazil court tries politicians over hit on Black councilwoman

-
 Senegal PM vows to double penalty for same-sex relations
Senegal PM vows to double penalty for same-sex relations
-
UK govt backs releasing documents tied to 'rude' ex-prince Andrew

-
 Novo Nordisk to slash prices of weightloss drugs in US
Novo Nordisk to slash prices of weightloss drugs in US
-
Welllage says Sri Lanka can rescue T20 World Cup campaign


Bora Biologics to Manufacture NYPOZI(TM) at San Diego Facility in Partnership with InvaGen Pharmaceuticals, a Cipla Group Company
SAN DIEGO, CA / ACCESS Newswire / September 25, 2025 / Bora Biologics, a leading contract development and manufacturing organization (CDMO) specializing in biologics, proudly announces its partnership with InvaGen Pharmaceuticals Inc., a wholly owned subsidiary of Cipla Limited, for the manufacture of NYPOZI™ (biosimilar to Neupogen®) at its FDA-registered facility in San Diego, California.
This collaboration signifies a major advancement in the biopharmaceutical sector, as NYPOZI™ is set to become the first FDA-approved biosimilar developed by a Taiwanese biopharma company to launch in the U.S. market. Bora Biologics will leverage its U.S. commercial manufacturing capabilities to support the production of this innovative therapy.
Bora Biologics' San Diego facility is equipped with advanced technology and rigorous GMP processes, enabling the efficient manufacture of high-quality biologics. This partnership underscores Bora Biologics' commitment to meeting the growing demands of the biopharmaceutical industry while ensuring the highest standards of quality and compliance.
"We are thrilled to be InvaGen's manufacturing partner for NYPOZI™ in the United States," said Stephen Lam, CEO of Bora Biologics. "Our FDA-registered facility is designed to meet the stringent requirements of biologics manufacturing, and we are dedicated to supporting this product's journey to market making more affordable medicine available to patients."
Marc Falkin, CEO, Cipla North America commented, "Our foray into the U.S. biosimilars signals a key milestone for Cipla. We are pleased to work with Bora Biologics to manufacture NYPOZI™ (biosimilar to Neupogen®) and improve access to high-quality affordable treatment. This further aligns with our purpose of 'Caring for Life' by making advanced therapies available to those in need, ultimately enhancing patient outcomes in the US."
This collaboration represents a significant milestone in Bora Biologics' mission to provide comprehensive manufacturing solutions and contribute to the advancement of life-saving therapies within its expanding GMP facility with two new 2000L bioreactors for biologics manufacturing opening in Q1 2026.
About Bora Biologics
Bora Biologics is a global CDMO offering agile, comprehensive end-to-end solutions for biopharma companies worldwide. With a proven track record of over 100 successful cGMP manufacturing batches, Bora Biologics leverages its state-of-the-art, FDA-registered facility in the U.S. and deep expertise in biologics development and manufacturing-including its own FDA-licensed and Health Canada approved product-to enhance time and cost efficiencies while ensuring effective pathways to market for its clients. Bora Biologics combines innovative early-phase development and late-stage manufacturing capabilities with the expertise and reputation of Bora Pharmaceuticals for flexible and scalable fill/finish services, including stability testing and final packaging of clinical and commercial products. Bora Biologics is a dba of Tanvex BioPharma USA, Inc.
Website
https://borabiologics.com
Media Contact
[email protected]
CDMO Inquiries
[email protected]
SOURCE: Bora Biologics
View the original press release on ACCESS Newswire
A.Malone--AMWN