
-
 Key to probe England's 'stag-do' drinking on Ashes beach break
Key to probe England's 'stag-do' drinking on Ashes beach break
-
Delayed US data expected to show solid growth in 3rd quarter

-
 Thunder bounce back to down Grizzlies, Nuggets sink Jazz
Thunder bounce back to down Grizzlies, Nuggets sink Jazz
-
Amazon says blocked 1,800 North Koreans from applying for jobs

-
 Trump says US needs Greenland 'for national security'
Trump says US needs Greenland 'for national security'
-
Purdy first 49er since Montana to throw five TDs as Colts beaten

-
 North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
-
Asian markets rally again as rate cut hopes bring Christmas cheer

-
 Australian state poised to approve sweeping new gun laws, protest ban
Australian state poised to approve sweeping new gun laws, protest ban
-
Trapped under Israeli bombardment, Gazans fear the 'new border'

-
 Families want answers a year after South Korea's deadliest plane crash
Families want answers a year after South Korea's deadliest plane crash
-
Myanmar's long march of military rule

-
 Disputed Myanmar election wins China's vote of confidence
Disputed Myanmar election wins China's vote of confidence
-
Myanmar junta stages election after five years of civil war

-
 Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
-
'Help me, I'm dying': inside Ecuador's TB-ridden gang-plagued prisons

-
 Australia's Cummins, Lyon out of fourth Ashes Test
Australia's Cummins, Lyon out of fourth Ashes Test
-
US singer Barry Manilow reveals lung cancer diagnosis

-
 'Call of Duty' co-creator Vince Zampella killed in car crash
'Call of Duty' co-creator Vince Zampella killed in car crash
-
Decentralized Masters Announced as the Best Crypto Course of 2025 (Courses on Cryptocurrency Ranked)

-
 Trump says would be 'smart' for Venezuela's Maduro to step down
Trump says would be 'smart' for Venezuela's Maduro to step down
-
Steelers' Metcalf suspended two games over fan outburst

-
 Salah, Foster take Egypt and South Africa to AFCON Group B summit
Salah, Foster take Egypt and South Africa to AFCON Group B summit
-
Napoli beat Bologna to lift Italian Super Cup

-
 Salah snatches added-time winner for Egypt after Zimbabwe scare
Salah snatches added-time winner for Egypt after Zimbabwe scare
-
Penalty king Jimenez strikes for Fulham to sink Forest

-
 Kansas City Chiefs confirm stadium move
Kansas City Chiefs confirm stadium move
-
Liverpool rocked by Isak blow after surgery on ankle injury

-
 US stocks push higher while gold, silver notch fresh records
US stocks push higher while gold, silver notch fresh records
-
Deadly clashes in Aleppo as Turkey urges Kurds not to be obstacle to Syria's stability

-
 Is the United States after Venezuela's oil?
Is the United States after Venezuela's oil?
-
Trump admin halts US offshore wind projects citing 'national security'

-
 Right wing urges boycott of iconic Brazilian flip-flops
Right wing urges boycott of iconic Brazilian flip-flops
-
From misfits to MAGA: Nicki Minaj's political whiplash

-
 Foster grabs South Africa winner against Angola in AFCON
Foster grabs South Africa winner against Angola in AFCON
-
Russia pledges 'full support' for Venezuela against US 'hostilities'

-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
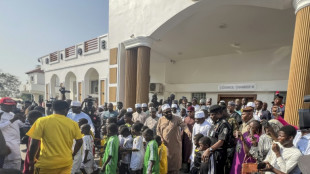 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing


Modular Medical Announces MODD1 Cartridge Line Validated for Human-Use Production
-Line has been in production at risk since early July
- Production to continue until late August when conversion to Pivot manufacturing will begin
SAN DIEGO, CA / ACCESS Newswire / August 4, 2025 / Modular Medical, Inc. (Nasdaq:MODD) ("Modular Medical" or the "Company"), an insulin delivery technology company with the first FDA-cleared patch pump designed specifically to target all adult "almost-pumpers" with its user-friendly and affordable design, announced that the MODD1 cartridge line has been validated for human-use production in the United States.
"This is an important milestone for scaling up our manufacturing infrastructure to support our commercial pilot for MODD1 and, eventually, our 3ml Pivot tubeless patch pump launch," stated Jeb Besser, CEO of Modular Medical. "While we encountered significant delays with the initial shipment of equipment for the manufacturing line to our contract manufacturing site in Mexico, all equipment for the controller line has been installed and validation is underway. We continue to target controller line validation in October for MODD1 with the commercial pilot for MODD1 to follow immediately thereafter."
The ability to scale production is a key differentiator in the pump space, especially given the much higher volumes required for a patch pump. Modular Medical's simple, low-cost platform was designed from the ground up for high volume manufacturing.
Forward-Looking Statements
This press release contains forward-looking statements that are made pursuant to the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements are subject to risks, trends, and uncertainties that could cause actual results to be materially different from the forward-looking statements contained in this press release, including but not limited to, the Company's ability to obtain the validations required to manufacture products for human use; the timing of achievement of such validations, if at all; the Company's ability to convert patients to use its pump products; and the occurrence of future events or circumstances, successful development of Modular Medical's proprietary technologies, whether the market will accept Modular Medical's products and services, anticipated consumer demand for the Company's products, whether Modular Medical can successfully manufacture its products at high volumes, general economic, and industry or political conditions in the United States or internationally, as well as other risk factors and business considerations described in Modular Medical's SEC filings, including its annual report on Form 10-K. Any forward-looking statements in this press release should be evaluated in light of these important risk factors. In addition, any forward-looking statements included in this press release represent Modular Medical's views only as of the date of its publication and should not be relied upon as representing its views as of any subsequent date. Modular Medical assumes no obligation to update these forward-looking statements, except as required by law.
About Modular Medical
Modular Medical, Inc. (Nasdaq: MODD) is a development-stage medical device company that intends to launch the next generation of insulin delivery technology. Using its patented technologies, the company seeks to eliminate the tradeoff between complexity and efficacy, thereby making top quality insulin delivery both affordable and simple to learn. Our mission is to improve access to the highest standard of glycemic control for people with diabetes taking it beyond "superusers" and providing "diabetes care for the rest of us."
Modular Medical was founded by Paul DiPerna, a seasoned medical device professional and microfluidics engineer. Prior to founding Modular Medical, Mr. DiPerna was the founder (in 2005) of Tandem Diabetes and invented and designed its t:slim insulin pump. More information is available at https://modular-medical.com.
All trademarks mentioned herein are the property of their respective owners.
CONTACT:
Jeb Besser
Chief Executive Officer
Modular Medical, Inc.
+1 (617) 399-1741
[email protected]
SOURCE: Modular Medical, Inc.
View the original press release on ACCESS Newswire
M.Fischer--AMWN