
-
 Key to probe England's 'stag-do' drinking on Ashes beach break
Key to probe England's 'stag-do' drinking on Ashes beach break
-
Delayed US data expected to show solid growth in 3rd quarter

-
 Thunder bounce back to down Grizzlies, Nuggets sink Jazz
Thunder bounce back to down Grizzlies, Nuggets sink Jazz
-
Amazon says blocked 1,800 North Koreans from applying for jobs

-
 Trump says US needs Greenland 'for national security'
Trump says US needs Greenland 'for national security'
-
Purdy first 49er since Montana to throw five TDs as Colts beaten

-
 North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
-
Asian markets rally again as rate cut hopes bring Christmas cheer

-
 Australian state poised to approve sweeping new gun laws, protest ban
Australian state poised to approve sweeping new gun laws, protest ban
-
Trapped under Israeli bombardment, Gazans fear the 'new border'

-
 Families want answers a year after South Korea's deadliest plane crash
Families want answers a year after South Korea's deadliest plane crash
-
Myanmar's long march of military rule

-
 Disputed Myanmar election wins China's vote of confidence
Disputed Myanmar election wins China's vote of confidence
-
Myanmar junta stages election after five years of civil war

-
 Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
-
'Help me, I'm dying': inside Ecuador's TB-ridden gang-plagued prisons

-
 Australia's Cummins, Lyon out of fourth Ashes Test
Australia's Cummins, Lyon out of fourth Ashes Test
-
US singer Barry Manilow reveals lung cancer diagnosis

-
 'Call of Duty' co-creator Vince Zampella killed in car crash
'Call of Duty' co-creator Vince Zampella killed in car crash
-
Decentralized Masters Announced as the Best Crypto Course of 2025 (Courses on Cryptocurrency Ranked)

-
 Trump says would be 'smart' for Venezuela's Maduro to step down
Trump says would be 'smart' for Venezuela's Maduro to step down
-
Steelers' Metcalf suspended two games over fan outburst

-
 Salah, Foster take Egypt and South Africa to AFCON Group B summit
Salah, Foster take Egypt and South Africa to AFCON Group B summit
-
Napoli beat Bologna to lift Italian Super Cup

-
 Salah snatches added-time winner for Egypt after Zimbabwe scare
Salah snatches added-time winner for Egypt after Zimbabwe scare
-
Penalty king Jimenez strikes for Fulham to sink Forest

-
 Kansas City Chiefs confirm stadium move
Kansas City Chiefs confirm stadium move
-
Liverpool rocked by Isak blow after surgery on ankle injury

-
 US stocks push higher while gold, silver notch fresh records
US stocks push higher while gold, silver notch fresh records
-
Deadly clashes in Aleppo as Turkey urges Kurds not to be obstacle to Syria's stability

-
 Is the United States after Venezuela's oil?
Is the United States after Venezuela's oil?
-
Trump admin halts US offshore wind projects citing 'national security'

-
 Right wing urges boycott of iconic Brazilian flip-flops
Right wing urges boycott of iconic Brazilian flip-flops
-
From misfits to MAGA: Nicki Minaj's political whiplash

-
 Foster grabs South Africa winner against Angola in AFCON
Foster grabs South Africa winner against Angola in AFCON
-
Russia pledges 'full support' for Venezuela against US 'hostilities'

-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
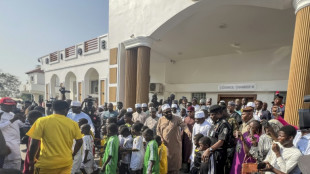 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing


Phase 1 Clinical Ttrial of HIV Vaccine Starts in Africa to Evaluate Immune Responses to Highly Networked HIV T-Cell Epitopes
Led by a team of African researchers, first doses of the novel T cell-inducing GRAdHIVNE1 vaccine candidate have been given.
HARARE, ZIMBABWE, ROME, ITALY, NEW YORK, NY, AND CAMBRIDGE, MA / ACCESS Newswire / August 4, 2025 / The Mutala Trust, ReiThera Srl (ReiThera), the Ragon Institute of Mass General Brigham, MIT, and Harvard (the Ragon Institute), and IAVI are pleased to announce that the first doses of an investigational HIV vaccine candidate have been administered. The vaccine candidate, Gorilla Adenovirus Vectored HIV Networked Epitopes Vaccine (GRAdHIVNE1), was first administered on July 28, 2025, at the Mutala Trust clinical trial site in Harare, Zimbabwe. This effort is made possible by a global collaboration and a team of African principal investigators who will lead the clinical research in South Africa and Zimbabwe (listed below). Vaccine immunogenicity will also be assessed locally by a network of state-of-the-art African research institutes: Cape Town HVTN Immunology Laboratory in Cape Town, African Health Research Institute in Durban, and the National Institute for Communicable Diseases in Johannesburg, South Africa.
This Phase 1, first-in-human clinical trial will enroll approximately 120 healthy adults aged 18-50 years, including 48 people living with HIV who are virally suppressed on antiretroviral therapy (ART). The trial is designed to assess the safety and immunogenicity of the vaccine candidate in people living with and without HIV. Participants will receive either one or two doses of the investigational vaccine or a placebo and will be monitored over a period of 19 months for safety and immune responses.
This clinical trial, IAVI C114, is sponsored by IAVI. GRAdHIVNE1 has been made possible by a collaborative effort. ReiThera developed the GRAd viral vector platform and manufactured the vaccine candidate, while the Ragon Institute designed the immunogen using novel strategies to identify protective HIV epitopes and facilitate their targeting by T cells. This clinical program is funded by the Gates Foundation.
The IAVI C114 clinical trial is taking place at three clinical trial sites: the Mutala Trust Clinical Trial Site, in Harare, Zimbabwe; the Desmond Tutu Health Foundation (DTHF), in Cape Town, South Africa; and the Africa Health Research Institute (AHRI), in Durban, South Africa. To determine the vaccine candidate's potential for relevance in sub-Saharan Africa, where disease burden is greatest, it is essential that the candidate be tested within communities affected by the epidemic.
"This is a landmark moment for South Africa, Zimbabwe, and the continent. It shows the power of true partnership: IAVI's sponsorship, ReiThera's GRAd technology, the Ragon Institute's innovative immunogen built on decades of science, and African investigators co-leading every phase of the trial. We are edging closer to an HIV vaccine, made possible by global collaboration, with clinical trials conducted in Africa, for Africa, and for the world." said Dr. Tariro Makadzange, Clinical Trial Lead, Mutala Trust.
"This trial represents the future of vaccine development, rooted in Africa, built through global partnerships, and designed for the communities most affected by HIV," said Dr. Vincent Muturi-Kioi, HIV Vaccines Product Development Team Lead at IAVI.
The vaccine candidate is designed to engage the immune system to recognize and target critical structural regions of HIV using a clinically validated, potent, T cell-inducing GRAd vector. This approach will be evaluated to assess the ability of the vaccine candidate to direct strong CD8+ T cell immune responses towards these vulnerable viral regions.
"We are thrilled to be moving insights from our long-term studies of spontaneous elite controllers of HIV toward the development of GRAdHIVNE1 and its testing in Africa. We are truly grateful to the network of global and African partners that have come together to make the IAVI C114 trial a reality," said Dr. Gaurav Gaiha, Associate Professor of Medicine at Harvard Medical School and Principal Investigator at the Ragon Institute of Mass General Brigham, MIT, and Harvard.
Because CD8+ T cells induced by this vaccine hold promise for targeting HIV-infected cells, this clinical trial will also assess the safety and immune response in people living with HIV. These data will be used to assess the suitability of the vaccine candidate for the development of investigational HIV therapeutic and curative interventions.
"We are enormously pleased with the launch of this Phase 1 trial representing the result of a successful global partnership," said Stefano Colloca, CEO and co-Founder of ReiThera. "This candidate HIV vaccine, built on our GRAd platform, holds great promise to trigger a strong CD8 response targeting vulnerable viral regions."
Principal Investigators Leading Clinical Trial Sites:
Tariro Makadzange, Mutala Trust
Theodorah Rirhandzu Ndzhukule, DTHF
Limakatso Lebina, AHRI
About IAVI
IAVI is a global nonprofit scientific organization that works to develop vaccines and antibodies to prevent HIV and other infectious diseases, with a focus on innovation and equitable access. IAVI is the sponsor of this trial. Read more at www.iavi.org.
IAVI media contact
Heather Teixeira
[email protected]
About Mutala Trust
Mutala Trust is founding member of Africa Clinical Research Network (ACRN) and is a site based in Harare, Zimbabwe. It is known for conducting high-quality, ethically sound clinical trials addressing diseases that affect African communities. Mutala is the clinical lead site for the study.
Mutala Trustmedia contact
About ReiThera Srl.
ReiThera Srl, an Italian CDMO specializing in technology and process development as well as GMP manufacturing of viral vectors for genetic vaccines and advanced therapies, is the developer and owner of the GRAd platform used for this HIV vaccine.
ReiThera media contact
About the Ragon Institute of Mass General Brigham, MIT, and Harvard
The Ragon Institute of Mass General Brigham, MIT, and Harvard was established with a collaborative scientific mission among these institutions that brings scientists, clinicians and engineers together to harness the immune system to combat and cure human disease. They contributed to vaccine design. For more information, visit www.ragoninstitute.org
Ragon Institutemedia contact
SOURCE: IAVI
View the original press release on ACCESS Newswire
P.Costa--AMWN
