
-
 Key to probe England's 'stag-do' drinking on Ashes beach break
Key to probe England's 'stag-do' drinking on Ashes beach break
-
Delayed US data expected to show solid growth in 3rd quarter

-
 Thunder bounce back to down Grizzlies, Nuggets sink Jazz
Thunder bounce back to down Grizzlies, Nuggets sink Jazz
-
Amazon says blocked 1,800 North Koreans from applying for jobs

-
 Trump says US needs Greenland 'for national security'
Trump says US needs Greenland 'for national security'
-
Purdy first 49er since Montana to throw five TDs as Colts beaten

-
 North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
-
Asian markets rally again as rate cut hopes bring Christmas cheer

-
 Australian state poised to approve sweeping new gun laws, protest ban
Australian state poised to approve sweeping new gun laws, protest ban
-
Trapped under Israeli bombardment, Gazans fear the 'new border'

-
 Families want answers a year after South Korea's deadliest plane crash
Families want answers a year after South Korea's deadliest plane crash
-
Myanmar's long march of military rule

-
 Disputed Myanmar election wins China's vote of confidence
Disputed Myanmar election wins China's vote of confidence
-
Myanmar junta stages election after five years of civil war

-
 Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
-
'Help me, I'm dying': inside Ecuador's TB-ridden gang-plagued prisons

-
 Australia's Cummins, Lyon out of fourth Ashes Test
Australia's Cummins, Lyon out of fourth Ashes Test
-
US singer Barry Manilow reveals lung cancer diagnosis

-
 'Call of Duty' co-creator Vince Zampella killed in car crash
'Call of Duty' co-creator Vince Zampella killed in car crash
-
Decentralized Masters Announced as the Best Crypto Course of 2025 (Courses on Cryptocurrency Ranked)

-
 Trump says would be 'smart' for Venezuela's Maduro to step down
Trump says would be 'smart' for Venezuela's Maduro to step down
-
Steelers' Metcalf suspended two games over fan outburst

-
 Salah, Foster take Egypt and South Africa to AFCON Group B summit
Salah, Foster take Egypt and South Africa to AFCON Group B summit
-
Napoli beat Bologna to lift Italian Super Cup

-
 Salah snatches added-time winner for Egypt after Zimbabwe scare
Salah snatches added-time winner for Egypt after Zimbabwe scare
-
Penalty king Jimenez strikes for Fulham to sink Forest

-
 Kansas City Chiefs confirm stadium move
Kansas City Chiefs confirm stadium move
-
Liverpool rocked by Isak blow after surgery on ankle injury

-
 US stocks push higher while gold, silver notch fresh records
US stocks push higher while gold, silver notch fresh records
-
Deadly clashes in Aleppo as Turkey urges Kurds not to be obstacle to Syria's stability

-
 Is the United States after Venezuela's oil?
Is the United States after Venezuela's oil?
-
Trump admin halts US offshore wind projects citing 'national security'

-
 Right wing urges boycott of iconic Brazilian flip-flops
Right wing urges boycott of iconic Brazilian flip-flops
-
From misfits to MAGA: Nicki Minaj's political whiplash

-
 Foster grabs South Africa winner against Angola in AFCON
Foster grabs South Africa winner against Angola in AFCON
-
Russia pledges 'full support' for Venezuela against US 'hostilities'

-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
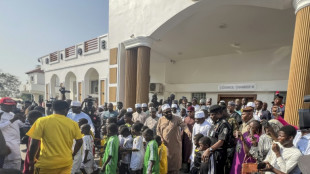 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing


CASI Pharmaceuticals Announces FDA Clearance of IND Application for CID-103 in Renal Allograft Antibody-Mediated Rejection (AMR)
CID-103 is a potential best-in-class, anti-CD38 monoclonal antibody; binds to unique CD38 epitope
Phase 1 study in adults with active and chronic active renal allograft AMR planned to initiate
AMR is a leading cause of kidney transplant loss, leading to dialysis and/or repeat renal transplant
SAN FRANCISCO, CA / ACCESS Newswire / August 4, 2025 / CASI Pharmaceuticals, Inc. (NASDAQ:CASI), a clinical-stage biopharmaceutical company focused on developing innovative therapies for patients with organ transplant rejection and autoimmune diseases, today announced FDA clearance of an IND application for CID-103, an anti-CD38 monoclonal antibody in adults with active and chronic active renal allograft antibody mediated rejection (AMR). The Phase 1 clinical trial is a dose-ranging and safety study evaluating the tolerability and efficacy of CID-103 in patients with AMR.
"Antibody-mediated rejection remains a significant challenge in kidney transplantation, with limited safe and effective treatment options currently available for those patients whose disease that has progressed," said Alex Zukiwski, M.D., Chief Medical Officer of CASI. "New therapeutic treatment options are urgently needed for patients with resistant AMR."
"This IND for CID-103 is a significant milestone in our mission at CASI to develop innovative therapies for patients with organ transplant rejection and autoimmune diseases," said David Cory, CEO of CASI. "We look forward to providing guidance in the future regarding the CID-103 development program."
About CID-103
CID-103 is a fully human IgG1, potentially best-in-class, anti-CD38 monoclonal antibody recognizing a unique epitope that has demonstrated an encouraging pre-clinical efficacy and safety profile compared to other anti-CD38 monoclonal antibodies, and for which CASI owns exclusive global rights. A body of evidence demonstrates the therapeutic promise of targeting CD38 in autoimmune diseases and organ transplant rejection. The CID-103 IND/CTA for immune thrombocytopenic purpura (ITP) has been approved by FDA and Chinese Health Authority and is actively recruiting and dosing patients. FDA has approved the IND application for renal allograft antibody-mediated rejection (AMR). The Company plans to initiate a Phase 1 study in adults with active and chronic active renal allograft AMR.
About CASI Pharmaceuticals
CASI Pharmaceuticals, Inc. is a biopharmaceutical company focused on developing and commercializing innovative therapeutics and pharmaceutical products in China, the U.S., and throughout the world. The Company is focused on acquiring, developing, and commercializing products that augment its focus on hematology oncology therapeutics and therapeutics for organ transplant rejection and autoimmune disease, as well as other areas of unmet medical need. The Company intends to execute its plan to become an industry leader by launching medicines in the Greater China market, leveraging the Company's China-based regulatory and commercial competencies and its global drug development expertise. The Company's operations in China are conducted through its wholly-owned subsidiary, CASI Pharmaceuticals (China) Co., Ltd., located in Beijing, China. More information on CASI is available at www.casipharmaceuticals.com.
Forward Looking Statements
This announcement contains forward-looking statements. These statements are made under the "safe harbor" provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as "will," "expects," "anticipates," "future," "intends," "plans," "believes," "estimates," "confident" and similar statements. Among other things, the business outlook and quotations from management in this announcement, as well as the Company's strategic and operational plans, contain forward-looking statements. The Company may also make written or oral forward-looking statements in its periodic reports to the U.S. Securities and Exchange Commission (the "SEC"), in its annual report to shareholders, in press releases and other written materials and in oral statements made by its officers, directors or employees to third parties. Statements that are not historical facts, including statements about the Company's beliefs and expectations, are forward-looking statements. Forward-looking statements involve inherent risks and uncertainties. A number of factors could cause actual results to differ materially from those contained in any forward-looking statement, including but not limited to the following: uncertainties related to the possibility that the transaction for the divestiture of certain assets in China (the "Transaction") will not occur as planned if events arise that result in the termination of the Equity and Assets Transfer Agreement, or if one or more of the various closing conditions to the Transaction are not satisfied or waived; the possibility that our plan with respect to our business operations after the consummation of the Transaction can be implemented successfully; our recurring operating losses have raised substantial doubt regarding our ability to continue as a going concern; the possibility that we may be delisted from trading on The Nasdaq Capital Market if we fail to satisfy applicable continued listing standards; the volatility in the market price of our ordinary shares; the risk of substantial dilution of existing shareholders in future share issuances; the difficulty of executing our business strategy on a global basis including China; our inability to enter into strategic partnerships for the development, commercialization, manufacturing and distribution of our proposed product candidates or future candidates; legal or regulatory developments in China that adversely affect our ability to operate in China; our lack of experience in manufacturing products and uncertainty about our resources and capabilities to do so on a clinical or commercial scale; risks relating to the commercialization, if any, of our products and proposed products (such as marketing, safety, regulatory, patent, product liability, supply, competition and other risks); our inability to predict when or if our product candidates will be approved for marketing by the U.S. Food and Drug Administration, European Medicines Agency, PRC National Medical Products Administration, or other regulatory authorities; our inability to receive approval for renewal of license of our existing products; the risks relating to the need for additional capital and the uncertainty of securing additional funding on favorable terms; the risks associated with our product candidates, and the risks associated with our other early-stage products under development; the risk that result in preclinical and clinical models are not necessarily indicative of clinical results; uncertainties relating to preclinical and clinical trials, including delays to the commencement of such trials; our ability to protect our intellectual property rights; the lack of success in the clinical development of any of our products; and our dependence on third parties; the risks related to our dependence on Juventas to conduct the clinical development of CNCT19 and to partner with us to co-market CNCT19; risks related to our dependence on Juventas to ensure the patent protection and prosecution for CNCT19; the risk related to the Company's ongoing development of and regulatory application for CID-103 with respect to the treatment of antibody-mediated rejection for organ transplant and the license arrangements of CID-103; risks relating to interests of our largest shareholder and our Chairman and CEO that differ from our other shareholders; risks related to the development of a new manufacturing facility by CASI Pharmaceuticals (Wuxi) Co., Ltd. and risks related to our disagreement with Acrotech with respect to the termination of agreements regarding EVOMELA®. Further information regarding these and other risks is included in the Company's filings with the SEC. All information provided herein is as of the date of this announcement, and the Company undertakes no obligation to update any forward-looking statement, except as required under applicable law. We caution readers not to place undue reliance on any forward-looking statements contained herein.
EVOMELA® is proprietary to Acrotech Biopharma Inc. and its affiliates. FOLOTYN® is proprietary to Acrotech Biopharma Inc and its affiliates. The Company is currently involved in disputes and legal proceedings related to certain pipeline products, including EVOMELA® and CNCT-19.Please refer to the Company's earlier SEC filing for further information.
COMPANY CONTACT:
Ingrid Choong, PhD
650-619-6115
[email protected]
SOURCE: CASI Pharmaceuticals
View the original press release on ACCESS Newswire
J.Oliveira--AMWN
