
-
 Key to probe England's 'stag-do' drinking on Ashes beach break
Key to probe England's 'stag-do' drinking on Ashes beach break
-
Delayed US data expected to show solid growth in 3rd quarter

-
 Thunder bounce back to down Grizzlies, Nuggets sink Jazz
Thunder bounce back to down Grizzlies, Nuggets sink Jazz
-
Amazon says blocked 1,800 North Koreans from applying for jobs

-
 Trump says US needs Greenland 'for national security'
Trump says US needs Greenland 'for national security'
-
Purdy first 49er since Montana to throw five TDs as Colts beaten

-
 North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
North Korea's Kim tours hot tubs, BBQ joints at lavish new mountain resort
-
Asian markets rally again as rate cut hopes bring Christmas cheer

-
 Australian state poised to approve sweeping new gun laws, protest ban
Australian state poised to approve sweeping new gun laws, protest ban
-
Trapped under Israeli bombardment, Gazans fear the 'new border'

-
 Families want answers a year after South Korea's deadliest plane crash
Families want answers a year after South Korea's deadliest plane crash
-
Myanmar's long march of military rule

-
 Disputed Myanmar election wins China's vote of confidence
Disputed Myanmar election wins China's vote of confidence
-
Myanmar junta stages election after five years of civil war

-
 Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
Ozempic Meals? Restaurants shrink portions to match bite-sized hunger
-
'Help me, I'm dying': inside Ecuador's TB-ridden gang-plagued prisons

-
 Australia's Cummins, Lyon out of fourth Ashes Test
Australia's Cummins, Lyon out of fourth Ashes Test
-
US singer Barry Manilow reveals lung cancer diagnosis

-
 'Call of Duty' co-creator Vince Zampella killed in car crash
'Call of Duty' co-creator Vince Zampella killed in car crash
-
Decentralized Masters Announced as the Best Crypto Course of 2025 (Courses on Cryptocurrency Ranked)

-
 Trump says would be 'smart' for Venezuela's Maduro to step down
Trump says would be 'smart' for Venezuela's Maduro to step down
-
Steelers' Metcalf suspended two games over fan outburst

-
 Salah, Foster take Egypt and South Africa to AFCON Group B summit
Salah, Foster take Egypt and South Africa to AFCON Group B summit
-
Napoli beat Bologna to lift Italian Super Cup

-
 Salah snatches added-time winner for Egypt after Zimbabwe scare
Salah snatches added-time winner for Egypt after Zimbabwe scare
-
Penalty king Jimenez strikes for Fulham to sink Forest

-
 Kansas City Chiefs confirm stadium move
Kansas City Chiefs confirm stadium move
-
Liverpool rocked by Isak blow after surgery on ankle injury

-
 US stocks push higher while gold, silver notch fresh records
US stocks push higher while gold, silver notch fresh records
-
Deadly clashes in Aleppo as Turkey urges Kurds not to be obstacle to Syria's stability

-
 Is the United States after Venezuela's oil?
Is the United States after Venezuela's oil?
-
Trump admin halts US offshore wind projects citing 'national security'

-
 Right wing urges boycott of iconic Brazilian flip-flops
Right wing urges boycott of iconic Brazilian flip-flops
-
From misfits to MAGA: Nicki Minaj's political whiplash

-
 Foster grabs South Africa winner against Angola in AFCON
Foster grabs South Africa winner against Angola in AFCON
-
Russia pledges 'full support' for Venezuela against US 'hostilities'

-
 Spotify says piracy activists hacked its music catalogue
Spotify says piracy activists hacked its music catalogue
-
Winter Olympics organisers resolve snow problem at ski site

-
 Fuming Denmark summons US ambassador over Greenland envoy
Fuming Denmark summons US ambassador over Greenland envoy
-
UK's street artist Banksy unveils latest mural in London

-
 Rugby players lose order challenge in brain injury claim
Rugby players lose order challenge in brain injury claim
-
UK singer Chris Rea dies at 74, days before Christmas

-
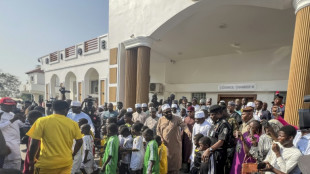 Last of kidnapped Nigerian pupils handed over, government says
Last of kidnapped Nigerian pupils handed over, government says
-
Zambia strike late to hold Mali in AFCON opener

-
 Outcry follows CBS pulling program on prison key to Trump deportations
Outcry follows CBS pulling program on prison key to Trump deportations
-
Sri Lanka cyclone caused $4.1 bn damage: World Bank

-
 Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
Billionaire Ellison offers personal guarantee for son's bid for Warner Bros
-
Tech stocks lead Wall Street higher, gold hits fresh record

-
 Telefonica to shed around 5,500 jobs in Spain
Telefonica to shed around 5,500 jobs in Spain
-
McCullum wants to stay as England coach despite Ashes drubbing


Onco-Innovations Advances Progress Towards First-in-Human Trial with Avance Clinical Start-Up Agreement
VANCOUVER, BC / ACCESS Newswire / August 4, 2025 / Onco-Innovations Limited (CBOE CA:ONCO)(OTCQB:ONNVF)(Frankfurt:W1H)(WKN:A3EKSZ) ("Onco" or the "Company") is pleased to announce it has entered into a Start-Up Agreement with Avance Clinical Pty Ltd ("Avance"), a leading contract research organization, to initiate key regulatory and clinical documentation, such as the scientific summaries and trial plans required to eventually apply for approvals for human testing, for the Company's lead candidate, the exclusively licensed Polynucleotide Kinase Phosphatase (PNKP) inhibitor (the "Technology"), a first-in-class, polymer-encapsulated therapy. The work is expected to lay the groundwork for regulatory clearance and clinical trial readiness.
Under the agreement, Avance will provide expert clinical and regulatory support to Onco as the Company pursues trials targeting advanced-stage cancers with PTEN1 or SHP-12 deficiency. Deliverables include the development of an Investigator's Brochure summarizing the drug's preclinical performance, the authorship of a detailed clinical trial protocol, and independent medical and statistical review related to a Phase I clinical study. These documents are essential prerequisites for regulatory agencies to evaluate the safety and scientific merit of an investigational therapy.
Additionally, Avance will perform a nonclinical gap analysis, reviewing existing data to identify any remaining preclinical work required for a future Investigational New Drug (IND) application. The agreement also covers the planning and preparation of a pre-IND meeting with the with the U.S. Food and Drug Administration (FDA), a critical regulatory milestone aimed at aligning Onco's trial design with agency expectations and reducing development risk.
"This collaboration with Avance is another example of our progress as we continue to move towards discovery to clinical development. It's about turning promising science into a real clinical opportunity for patients. Their regulatory and operational expertise will be essential in shaping a trial that is not only scientifically sound but also built for execution. We're excited to enter this collaboration with Avance," stated Thomas O'Shaughnessy, CEO of Onco-Innovations.
About Avance Clinical
Avance Clinical is a full-service Contract Research Organization (CRO) with clinical operations spanning Australia, New Zealand, Asia, North America, and Europe.3 The company is recognized for delivering high-quality clinical trials for international biotechs, with deep experience across oncology, rare diseases, CNS, cell and gene therapies, and infectious diseases.4 In the past five years, Avance has successfully completed over 60 trials in rare and orphan disease populations and more than 67 oncology studies globally, highlighting their strong specialty track record.5
About Onco-Innovations Limited
Onco-Innovations is a Canadian-based company dedicated to cancer research and treatment, specializing in oncology. Onco's mission is to pursue the prevention and treatment of cancer through pioneering research and innovative solutions. The company has secured an exclusive worldwide license to patented technology that targets solid tumours.
ON BEHALF OF ONCO-INNOVATIONS LIMITED,
"Thomas O'Shaughnessy"
Chief Executive Officer
For more information, please contact:
Thomas O'Shaughnessy
Chief Executive Officer
Tel: + 1 888 261 8055
[email protected]
Forward-Looking Statements Caution. This news release contains forward-looking statements, including in relation to the benefits expected to be realized under the agreement with Nucro-Technics, and the Company's ability to move forward with its plans for regulatory approvals and the conduct of human and other further testing of its Technologies, the prospects of the Company, and the Company's business and plans generally, and other statements that are not historical facts. Forward-looking statements are often identified by terms such as "will", "may", "potential", "should", "anticipate", "expects" and similar expressions. All statements other than statements of historical fact, included in this release are forward-looking statements that involve risks and uncertainties. There can be no assurance that such statements will prove to be accurate and actual results and future events could differ materially from those anticipated in such statements. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management at the time of preparation, may prove to be incorrect and actual results may differ materially from those anticipated. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date of this news release and the Company will update or revise publicly any of the included forward-looking statements as expressly required by applicable law.
1 PTEN is a tumor suppressor gene that helps prevent uncontrolled cell growth by regulating signals that control cell division and survival. Mutations or loss of PTEN function can disrupt this balance and lead to tumor formation. See PTEN gene - MedlinePlus Genetics: https://medlineplus.gov/genetics/gene/pten
2 SHP-1 is a protein that helps control cell signaling, acting as a "brake" on pathways that promote cell growth and survival. When SHP-1 is missing or inactive, these signals can become overactive, contributing to cancer development. See SHP-1 - National Center for Biotechnology Information (NCBI): https://www.ncbi.nlm.nih.gov/gene/5777
5 https://www.avancecro.com/therapeutic-areas/rare-disease-orphan-drug
SOURCE: Onco-Innovations Limited
View the original press release on ACCESS Newswire
Th.Berger--AMWN
