
-
 Germany says China promised 'reliable' rare earth supply
Germany says China promised 'reliable' rare earth supply
-
Spanish PM urges defence of democracy, 50 years after Franco death

-
 Israel launches fresh strikes on Gaza as Qatar fears for truce
Israel launches fresh strikes on Gaza as Qatar fears for truce
-
UN celebrates youth activists using tech for good

-
 AI's blind spot: tools fail to detect their own fakes
AI's blind spot: tools fail to detect their own fakes
-
US health agency edits official website to reflect anti-vax views

-
 US unemployment up even as hiring beat expectations in delayed report
US unemployment up even as hiring beat expectations in delayed report
-
US honors conservative titan Cheney, with Trump off guest list

-
 Nigerian court jails Biafran separatist leader Kanu for life for 'terrorism'
Nigerian court jails Biafran separatist leader Kanu for life for 'terrorism'
-
Spain fight back against Czech Republic to reach Davis Cup semis

-
 UN chief calls for 'ambitious compromise' at climate talks
UN chief calls for 'ambitious compromise' at climate talks
-
Comet sparks scientific fascination, online furor over 'alien' origins

-
 German Christmas market opens year after deadly car attack
German Christmas market opens year after deadly car attack
-
Stocks rise as Nvidia overshadows US jobs report

-
 Irish veterans Ringrose and van der Flier return for South Africa Test
Irish veterans Ringrose and van der Flier return for South Africa Test
-
Vietnam flooding submerges homes, kills 41, after relentless rain

-
 Nigeria convicts Biafran separatist leader Kanu for 'terrorism'
Nigeria convicts Biafran separatist leader Kanu for 'terrorism'
-
Varney misses Italy's Chile Test with rib fracture

-
 'Exciting prospect' Gordon recalled by Australia coach Schmidt
'Exciting prospect' Gordon recalled by Australia coach Schmidt
-
US unemployment up even as hiring beats expectations in delayed report

-
 Nigeria convicts Biafran separatist leader Nnamdi Kanu for 'terrorism'
Nigeria convicts Biafran separatist leader Nnamdi Kanu for 'terrorism'
-
UN nuclear watchdog demands Iran open up bombed nuclear sites

-
 Walmart earnings beat expectations as shoppers seek savings
Walmart earnings beat expectations as shoppers seek savings
-
South Africa back to full strength for 'colossal challenge' of Irish

-
 Greenpeace says clothes sold by Shein break EU chemicals rules
Greenpeace says clothes sold by Shein break EU chemicals rules
-
Italy to face Northern Ireland in 2026 World Cup playoffs

-
 Inexperienced Gordon recalled by Australia coach Schmidt
Inexperienced Gordon recalled by Australia coach Schmidt
-
Walmart lifts outlook in quarterly results with e-commerce boost

-
 EU moves to bar 'green' labels for fossil fuel investments
EU moves to bar 'green' labels for fossil fuel investments
-
Lufthansa enters race for TAP stake against Air France-KLM

-
 Daily pill helps people lose 10% of weight in 18 months: study
Daily pill helps people lose 10% of weight in 18 months: study
-
Barca go 'back to the future' for renovated Camp Nou reopening

-
 Youth activist turning trauma into treatment in Lebanon
Youth activist turning trauma into treatment in Lebanon
-
The US plan for ending the Ukraine war: What do we know?

-
 Stocks mostly rise as Nvidia calms AI fears
Stocks mostly rise as Nvidia calms AI fears
-
Alldritt keeps France captaincy as Fickou returns against Australia

-
 Israel launches fresh strikes on Gaza, Qatar warns of escalation
Israel launches fresh strikes on Gaza, Qatar warns of escalation
-
Veteran Fickou returns for France against Australia

-
 Spain court orders Meta to compensate media for 'unfair competition'
Spain court orders Meta to compensate media for 'unfair competition'
-
Australia's Smith takes pre-Ashes swipe at 'Mastermind' Panesar

-
 Deaves to debut for Wales against New Zealand
Deaves to debut for Wales against New Zealand
-
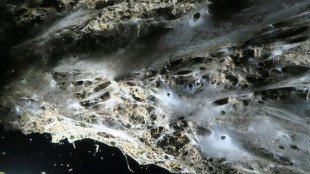
Giant spider web found in Greek-Albanian border cave: study
-
 Chinese woman who faked nationality to become Philippine mayor jailed for trafficking
Chinese woman who faked nationality to become Philippine mayor jailed for trafficking
-
New Zealand ring changes for Wales Test

-
 Most markets rise as Nvidia earnings override Fed rate concern
Most markets rise as Nvidia earnings override Fed rate concern
-
Vietnam flooding submerges homes, kills 16, after relentless rain

-
 The case of Africa's 'vanishing' carbon deals
The case of Africa's 'vanishing' carbon deals
-
Stokes tells England to 'write our own history' in Ashes

-
 Young Nepalis drive a new wave of voters and candidates
Young Nepalis drive a new wave of voters and candidates
-
Bangladesh's Mushfiqur joins elite club with ton in 100th Test

| CMSD | -0.35% | 23.67 | $ | |
| BCC | 1.4% | 68.175 | $ | |
| RIO | 0.01% | 69.44 | $ | |
| SCS | -0.19% | 15.7 | $ | |
| BTI | -0.05% | 54.714 | $ | |
| GSK | -0.36% | 46.175 | $ | |
| NGG | -0.24% | 75.91 | $ | |
| RBGPF | 2.47% | 79.04 | $ | |
| JRI | 0.19% | 13.275 | $ | |
| AZN | 0.04% | 89.03 | $ | |
| RYCEF | 0% | 14.15 | $ | |
| BCE | 0.15% | 22.825 | $ | |
| BP | 0.19% | 36.02 | $ | |
| VOD | -0.97% | 11.895 | $ | |
| RELX | -0.59% | 39.565 | $ | |
| CMSC | -0.72% | 23.5 | $ |

Ensysce Biosciences Receives Positive FDA Feedback on PF614 Manufacturing Approach
~ FDA Response Streamlines Path to Commercial Production of PF614~
SAN DIEGO, CA / ACCESS Newswire / November 20, 2025 / Ensysce Biosciences, Inc. (NASDAQ:ENSC) ("Ensysce" or the "Company"), a clinical-stage pharmaceutical company pioneering next-generation pain and central nervous system therapeutics designed to minimize abuse and overdose risk, today announced that the Food and Drug Administration (FDA or Agency) provided Written Responses to a meeting request.
Ensysce had requested guidance on its approach to the manufacture of PF614, wanting to understand the appropriateness of regulatory starting materials (RSMs) and specifications for PF614 drug substance and the RSMs.
In its written responses, the Agency agreed with all of Ensysce's proposed plans. These responses provide Ensysce with a direct path to commercial production of PF614, which is currently being initiated with its manufacturing partner, Purisys, LLC, a subsidiary of Noramco, LLC.
Dr. Jeff Millard, Chief Operating Officer of Ensysce who lead the CMC effort stated "We are extremely pleased with the FDA's feedback, which validates our approach and enables us to accelerate PF614's path to market. This milestone brings us closer to providing safer pain relief options for patients in need."
"The feedback from the FDA now provides us with a clear path to scaling our manufacture of PF614 for its commercial production," said Dr. Lynn Kirkpatrick, Chief Executive Officer of Ensysce. "PF614 is our TAAP™ oxycodone analogue and the leading product in our Next Generation Analgesic pipeline of TAAP™ and MPAR® products. We are exceptionally pleased that our meeting package was so well received and that we are aligned with the FDA requirements for producing this novel therapeutic that is designed to alleviate moderate to severe pain while providing what we believe are safer qualities for the general public who require this level of pain relief."
About Ensysce Biosciences
Ensysce Biosciences is a clinical-stage company with a goal of disrupting the analgesic landscape by introducing a new class of highly novel opioids for the treatment of severe pain. Leveraging its Trypsin-Activated Abuse Protection (TAAP™) and Multi-Pill Abuse Resistance (MPAR®) platforms, the Company is developing unique, tamper-proof treatment options for pain that minimize the risk of both drug abuse and overdose. Ensysce's products are anticipated to provide safer options to treat patients suffering from severe pain and assist in preventing deaths caused by medication abuse. For more information, please visit www.ensysce.com.
Forward-Looking Statements
Statements contained in this press release that are not purely historical may be deemed to be forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. Without limiting the foregoing, the use of words such as "may," "intends," "can," "might," "will," "expect," "plan," "possible," "believe" and other similar expressions are intended to identify forward-looking statements. The product candidates discussed are in clinic and not approved and there can be no assurance that the clinical programs will be successful in demonstrating safety and/or efficacy, that Ensysce will not encounter problems or delays in clinical development, or that any product candidate will ever receive regulatory approval or be successfully commercialized. All forward-looking statements are based on estimates and assumptions by Ensysce's management that, although Ensysce believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Ensysce expected. In addition, Ensysce's business is subject to additional risks and uncertainties, including among others, possible NASDAQ delisting, the initiation and conduct of preclinical studies and clinical trials; the timing and availability of data from preclinical studies and clinical trials; expectations for regulatory submissions and approvals; potential safety concerns related to, or efficacy of, Ensysce's product candidates; the availability or commercial potential of product candidates; continuation of government funding; the ability of Ensysce to fund its continued operations, including its planned clinical trials; the dilutive effect of stock issuances from our fundraising; and Ensysce's and its partners' ability to perform under their license, collaboration and manufacturing arrangements. These statements are also subject to a number of material risks and uncertainties that are described in Ensysce's most recent quarterly report on Form 10-Q and current reports on Form 8-K, available free of charge at the SEC's website at www.sec.gov. Any forward-looking statement speaks only as of the date on which it was made. Ensysce undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required under applicable law.
Ensysce Biosciences Company Contact:
Lynn Kirkpatrick, Ph.D.
Chief Executive Officer
(858) 263-4196
Ensysce Biosciences Investor Relations Contact:
Shannon Devine
MZ North America
Main: 203-741-8811
[email protected]
SOURCE: Ensysce Biosciences Inc.
View the original press release on ACCESS Newswire
C.Garcia--AMWN